Corrosion inhibition of a novel antihistamine-based compound for mild steel in hydrochloric acid solution: experimental and computational studies
- PMID: 35927311
- PMCID: PMC9352695
- DOI: 10.1038/s41598-022-17589-y
Corrosion inhibition of a novel antihistamine-based compound for mild steel in hydrochloric acid solution: experimental and computational studies
Abstract
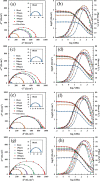
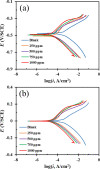
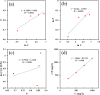
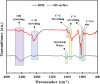
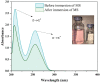
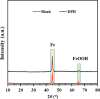
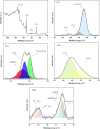
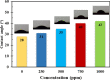
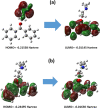
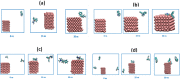
Focused on the assessment of the diphenhydramine hydrochloride (DPH) capabilities as an alternative to conventional and harmful industrial corrosion inhibitors, electrochemical techniques were employed. The optimum concentration of 1000 ppm was determined by molecular simulation and validated through electrochemical experiments. The results acquired from the electrochemical impedance spectroscopy (EIS) study showed that DPH at a concentration of 1000 ppm has a corrosion efficiency of 91.43% after 6 h immersion. The DPH molecules' orientation on the surface was assessed based on EIS predicting horizontal adsorption on the surface. Molecular simulations were done to explore the adsorption mechanism of DPH. The DPH molecules' orientation on the surface was also assessed based on computational studies confirming the horizontal adsorption predicted by EIS.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Prabakaran M, Kim SH, Hemapriya V, Chung IM. Tragia plukenetii extract as an eco-friendly inhibitor for mild steel corrosion in HCl 1 M acidic medium. Res. Chem. Intermed. 2016;42:3703–3719. doi: 10.1007/s11164-015-2240-x. - DOI
-
- Shahini MH, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B. Superior inhibition action of the Mish Gush (MG) leaves extract toward mild steel corrosion in HCl solution: Theoretical and electrochemical studies. J. Mol. Liq. 2021;332:115876. doi: 10.1016/j.molliq.2021.115876. - DOI
-
- Alibakhshi E, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 2018;255:185–198. doi: 10.1016/j.molliq.2018.01.144. - DOI
-
- Farsak M, Keles H, Keles M. A new corrosion inhibitor for protection of low carbon steel in HCl solution. Corros. Sci. 2015;98:223–232. doi: 10.1016/j.corsci.2015.05.036. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

