Immune-based combination therapy to convert immunologically cold tumors into hot tumors: an update and new insights
- PMID: 35927312
- PMCID: PMC9889774
- DOI: 10.1038/s41401-022-00953-z
Immune-based combination therapy to convert immunologically cold tumors into hot tumors: an update and new insights
Abstract
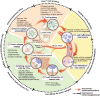
As a breakthrough strategy for cancer treatment, immunotherapy mainly consists of immune checkpoint inhibitors (ICIs) and other immunomodulatory drugs that provide a durable protective antitumor response by stimulating the immune system to fight cancer. However, due to the low response rate and unique toxicity profiles of immunotherapy, the strategies of combining immunotherapy with other therapies have attracted enormous attention. These combinations are designed to exert potent antitumor effects by regulating different processes in the cancer-immunity cycle. To date, immune-based combination therapy has achieved encouraging results in numerous clinical trials and has received Food and Drug Administration (FDA) approval for certain cancers with more studies underway. This review summarizes the emerging strategies of immune-based combination therapy, including combinations with another immunotherapeutic strategy, radiotherapy, chemotherapy, anti-angiogenic therapy, targeted therapy, bacterial therapy, and stroma-targeted therapy. Here, we highlight the rationale of immune-based combination therapy, the biomarkers and the clinical progress for these immune-based combination therapies.
Keywords: biomarkers; combination therapy; immune checkpoint inhibitors; immunotherapy; neoplasms.
© 2022. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

