Colorimetric determination of carbidopa in anti-Parkinson drugs based on 4-hydroxy-3-methoxybenzaldazine formation by reaction with vanillin
- PMID: 35927364
- PMCID: PMC9436860
- DOI: 10.1007/s00216-022-04256-4
Colorimetric determination of carbidopa in anti-Parkinson drugs based on 4-hydroxy-3-methoxybenzaldazine formation by reaction with vanillin
Abstract
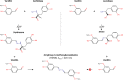
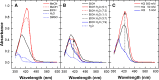
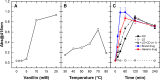
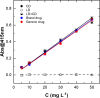
In this paper is reported the selective colorimetric detection and quantification of carbidopa, an inhibitor of aromatic amino acid decarboxylase, in the co-presence of levodopa as dopamine precursor in pharmaceutical formulations for the treatment of Parkinson's disease. The method is based on the selective condensation reaction between the hydrazine group from carbidopa and the formyl functional group of vanillin, a natural flavoring agent, in acidified alcoholic solution. The yellow color development (λmax ~ 420 nm) due to the formation of 4-hydroxy-3-methoxybenzaldazine (HMOB) was observed for carbidopa only, whereas levodopa, lacking the hydrazine group, did not color the solution, as expected. The calibration curves for two tablet formulations of levodopa in combination with carbidopa (4:1) were superimposable with levodopa/carbidopa (4:1), as well as carbidopa alone, in standard solution, i.e., the excipients and additives did not interfere with carbidopa determination, corresponding to a mean recovery about 105%. The linear dynamic range was between 5.00 and 50.0 mg L-1 with very good reproducibility within this range (CVav% about 3-4%) and very good sensitivity, with limits of quantification of about 1 mg L-1. The colorimetric method developed here is very simple, inexpensive, and effective for drug estimation and quality control of pharmaceutical formulations.
Keywords: Anti-Parkinson drugs; Benzaldazine; Carbidopa; Colorimetry; Levodopa; Vanillin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Quantitative Colorimetric Sensing of Carbidopa in Anti-Parkinson Drugs Based on Selective Reaction with Indole-3-Carbaldehyde.Sensors (Basel). 2023 Nov 13;23(22):9142. doi: 10.3390/s23229142. Sensors (Basel). 2023. PMID: 38005530 Free PMC article.
-
Melanochrome-based colorimetric assay for quantitative detection of levodopa in co-presence of carbidopa and its application to relevant anti-Parkinson drugs.Anal Bioanal Chem. 2022 Feb;414(4):1713-1722. doi: 10.1007/s00216-021-03804-8. Epub 2021 Nov 29. Anal Bioanal Chem. 2022. PMID: 34842945
-
Comparison of dopa decarboxylase inhibitor (carbidopa) combined with levodopa and levodopa alone in Parkinson's disease.Neurology. 1975 Oct;25(10):911-6. doi: 10.1212/wnl.25.10.911. Neurology. 1975. PMID: 1101099 Clinical Trial.
-
Entacapone/levodopa/carbidopa combination tablet: Stalevo.Drugs R D. 2003;4(5):310-1. doi: 10.2165/00126839-200304050-00006. Drugs R D. 2003. PMID: 12952501 Review.
-
Levodopa/Carbidopa Enteral Suspension: A Review in Advanced Parkinson's Disease.Drugs. 2019 Oct;79(15):1709-1718. doi: 10.1007/s40265-019-01201-1. Drugs. 2019. PMID: 31549300 Review.
Cited by
-
Quantitative Colorimetric Sensing of Carbidopa in Anti-Parkinson Drugs Based on Selective Reaction with Indole-3-Carbaldehyde.Sensors (Basel). 2023 Nov 13;23(22):9142. doi: 10.3390/s23229142. Sensors (Basel). 2023. PMID: 38005530 Free PMC article.
-
Sensing of Catecholamine in Human Urine Using a Simple Colorimetric Assay Based on Direct Melanochrome and Indolequinone Formation.Sensors (Basel). 2023 Apr 13;23(8):3971. doi: 10.3390/s23083971. Sensors (Basel). 2023. PMID: 37112313 Free PMC article.
References
-
- https://www.parkinson.org/Understanding-Parkinsons/Statistics Accessed 31 May 2022.
-
- Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;1670–83. 10.1001/jama.2014.3654. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

