Paracrine-mediated rejuvenation of aged mesenchymal stem cells is associated with downregulation of the autophagy-lysosomal pathway
- PMID: 35927427
- PMCID: PMC9293998
- DOI: 10.1038/s41514-022-00091-0
Paracrine-mediated rejuvenation of aged mesenchymal stem cells is associated with downregulation of the autophagy-lysosomal pathway
Abstract
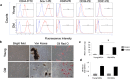
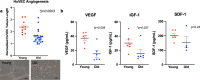
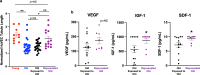
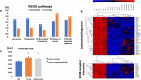
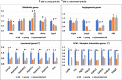
Age-related differences in stem-cell potency contribute to variable outcomes in clinical stem cell trials. To help understand the effect of age on stem cell potency, bone marrow-derived mesenchymal stem cells (MSCs) were isolated from young (6 weeks) and old (18-24 months) mice. HUVEC tubule formation (TF) induced by the old and young MSCs and ELISA of conditioned media were compared to one another, and to old MSCs after 7 d in indirect co-culture with young MSCs. Old MSCs induced less TF than did young (1.56 ± 0.11 vs 2.38 ± 0.17, p = 0.0003) and released lower amounts of VEGF (p = 0.009) and IGF1 (p = 0.037). After 7 d in co-culture with young MSCs, TF by the old MSCs significantly improved (to 2.09 ± 0.18 from 1.56 ± 0.11; p = 0.013), and was no longer different compared to TF from young MSCs (2.09 ± 0.18 vs 2.38 ± 0.17; p = 0.27). RNA seq of old MSCs, young MSCs, and old MSCs following co-culture with young MSCs revealed that the age-related differences were broadly modified by co-culture, with the most significant changes associated with lysosomal pathways. These results indicate that the age-associated decreased paracrine-mediated effects of old MSCs are improved following indirect co-culture with young MSC. The observed effect is associated with broad transcriptional modification, suggesting potential targets to both assess and improve the therapeutic potency of stem cells from older patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

