Single mutation makes Escherichia coli an insect mutualist
- PMID: 35927448
- PMCID: PMC9352592
- DOI: 10.1038/s41564-022-01179-9
Single mutation makes Escherichia coli an insect mutualist
Abstract
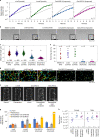
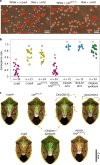
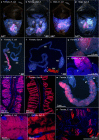
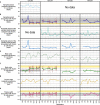
Microorganisms often live in symbiosis with their hosts, and some are considered mutualists, where all species involved benefit from the interaction. How free-living microorganisms have evolved to become mutualists is unclear. Here we report an experimental system in which non-symbiotic Escherichia coli evolves into an insect mutualist. The stinkbug Plautia stali is typically associated with its essential gut symbiont, Pantoea sp., which colonizes a specialized symbiotic organ. When sterilized newborn nymphs were infected with E. coli rather than Pantoea sp., only a few insects survived, in which E. coli exhibited specific localization to the symbiotic organ and vertical transmission to the offspring. Through transgenerational maintenance with P. stali, several hypermutating E. coli lines independently evolved to support the host's high adult emergence and improved body colour; these were called 'mutualistic' E. coli. These mutants exhibited slower bacterial growth, smaller size, loss of flagellar motility and lack of an extracellular matrix. Transcriptomic and genomic analyses of 'mutualistic' E. coli lines revealed independent mutations that disrupted the carbon catabolite repression global transcriptional regulator system. Each mutation reproduced the mutualistic phenotypes when introduced into wild-type E. coli, confirming that single carbon catabolite repression mutations can make E. coli an insect mutualist. These findings provide an experimental system for future work on host-microbe symbioses and may explain why microbial mutualisms are omnipresent in nature.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures











Comment in
-
Fast track to mutualism.Nat Microbiol. 2022 Aug;7(8):1104-1105. doi: 10.1038/s41564-022-01188-8. Nat Microbiol. 2022. PMID: 35927447 No abstract available.
Similar articles
-
Mechanisms Underpinning Morphogenesis of a Symbiotic Organ Specialized for Hosting an Indispensable Microbial Symbiont in Stinkbugs.mBio. 2023 Apr 25;14(2):e0052223. doi: 10.1128/mbio.00522-23. Epub 2023 Apr 5. mBio. 2023. PMID: 37017516 Free PMC article.
-
Host range of naturally and artificially evolved symbiotic bacteria for a specific host insect.mBio. 2024 Sep 11;15(9):e0134224. doi: 10.1128/mbio.01342-24. Epub 2024 Jul 31. mBio. 2024. PMID: 39082826 Free PMC article.
-
Regulation and remodeling of microbial symbiosis in insect metamorphosis.Proc Natl Acad Sci U S A. 2023 Oct 3;120(40):e2304879120. doi: 10.1073/pnas.2304879120. Epub 2023 Sep 28. Proc Natl Acad Sci U S A. 2023. PMID: 37769258 Free PMC article.
-
Riptortus pedestris and Burkholderia symbiont: an ideal model system for insect-microbe symbiotic associations.Res Microbiol. 2017 Apr;168(3):175-187. doi: 10.1016/j.resmic.2016.11.005. Epub 2016 Dec 10. Res Microbiol. 2017. PMID: 27965151 Review.
-
Insect symbionts as hidden players in insect-plant interactions.Trends Ecol Evol. 2012 Dec;27(12):705-11. doi: 10.1016/j.tree.2012.08.013. Epub 2012 Sep 15. Trends Ecol Evol. 2012. PMID: 22985943 Review.
Cited by
-
Plant pathogenesis: Toward multidimensional understanding of the microbiome.Imeta. 2023 Jul 16;2(3):e129. doi: 10.1002/imt2.129. eCollection 2023 Aug. Imeta. 2023. PMID: 38867927 Free PMC article. Review.
-
Fitness trade-offs and the origins of endosymbiosis.PLoS Biol. 2024 Apr 12;22(4):e3002580. doi: 10.1371/journal.pbio.3002580. eCollection 2024 Apr. PLoS Biol. 2024. PMID: 38607979 Free PMC article.
-
Mechanisms Underpinning Morphogenesis of a Symbiotic Organ Specialized for Hosting an Indispensable Microbial Symbiont in Stinkbugs.mBio. 2023 Apr 25;14(2):e0052223. doi: 10.1128/mbio.00522-23. Epub 2023 Apr 5. mBio. 2023. PMID: 37017516 Free PMC article.
-
Congruence between noise and plasticity in protein expression.Sci Rep. 2025 Jul 8;15(1):24529. doi: 10.1038/s41598-025-07594-2. Sci Rep. 2025. PMID: 40628805 Free PMC article.
-
Cyclic-di-GMP promotes bacteria-host association.Nat Microbiol. 2023 Oct;8(10):1758-1759. doi: 10.1038/s41564-023-01479-8. Nat Microbiol. 2023. PMID: 37679598 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

