The roles of extracellular vesicles in the immune system
- PMID: 35927511
- PMCID: PMC9361922
- DOI: 10.1038/s41577-022-00763-8
The roles of extracellular vesicles in the immune system
Abstract
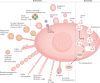
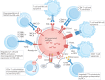
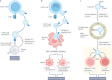
The twenty-first century has witnessed major developments in the field of extracellular vesicle (EV) research, including significant steps towards defining standard criteria for the separation and detection of EVs. The recent recognition that EVs have the potential to function as biomarkers or as therapeutic tools has attracted even greater attention to their study. With this progress in mind, an updated comprehensive overview of the roles of EVs in the immune system is timely. This Review summarizes the roles of EVs in basic processes of innate and adaptive immunity, including inflammation, antigen presentation, and the development and activation of B cells and T cells. It also highlights key progress related to deciphering the roles of EVs in antimicrobial defence and in allergic, autoimmune and antitumour immune responses. It ends with a focus on the relevance of EVs to immunotherapy and vaccination, drawing attention to ongoing or recently completed clinical trials that aim to harness the therapeutic potential of EVs.
© 2022. Springer Nature Limited.
Conflict of interest statement
E.I.B. is a member of the Advisory Board of Sphere Gene Therapeutics Inc. (Boston, MA, USA).
Figures

References
-
- Théry C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

