Three-dimensional models of the cervicovaginal epithelia to study host-microbiome interactions and sexually transmitted infections
- PMID: 35927516
- PMCID: PMC9419571
- DOI: 10.1093/femspd/ftac026
Three-dimensional models of the cervicovaginal epithelia to study host-microbiome interactions and sexually transmitted infections
Abstract
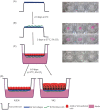
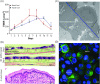
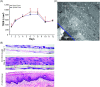
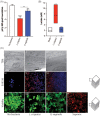
2D cell culture systems have historically provided controlled, reproducible means to analyze host-pathogen interactions observed in the human reproductive tract. Although inexpensive, straightforward, and requiring a very short time commitment, these models recapitulate neither the functionality of multilayered cell types nor the associated microbiome that occurs in a human. Animal models have commonly been used to recreate the complexity of human infections. However, extensive modifications of animal models are required to recreate interactions that resemble those in the human reproductive tract. 3D cell culture models have emerged as alternative means of reproducing vital elements of human infections at a fraction of the cost of animal models and on a scale that allows for replicative experiments. Here, we describe a new 3D model that utilizes transwells with epithelial cells seeded apically and a basolateral extracellular matrix (ECM)-like layer. The model produced tissues with morphologic and physiological resemblance to human cervical and vaginal epithelia, including mucus levels produced by cervical cells. Infection by Chlamydia trachomatis and Neisseria gonorrhoeae was demonstrated, as well as the growth of bacterial species observed in the human vaginal microbiota. This enabled controlled mechanistic analyses of the interactions between host cells, the vaginal microbiota, and STI pathogens. Affordable and semi high-throughput 3D models of the cervicovaginal epithelia that are physiologically relevant by sustaining vaginal bacterial colonization, and facilitate studies of chlamydial and gonococcal infections.
Keywords: 3D model; cervicovaginal epithelium; microbiome; sexually transmitted infections.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Figures


Similar articles
-
The Cervicovaginal Microbiota-Host Interaction Modulates Chlamydia trachomatis Infection.mBio. 2019 Aug 13;10(4):e01548-19. doi: 10.1128/mBio.01548-19. mBio. 2019. PMID: 31409678 Free PMC article.
-
The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections: a systematic review and meta-analysis.Clin Microbiol Infect. 2019 Jan;25(1):35-47. doi: 10.1016/j.cmi.2018.04.019. Epub 2018 May 3. Clin Microbiol Infect. 2019. PMID: 29729331 Free PMC article.
-
Subsequent sexually transmitted infections among adolescent women with genital infection due to Chlamydia trachomatis, Neisseria gonorrhoeae, or Trichomonas vaginalis.Sex Transm Dis. 1999 Jan;26(1):26-32. doi: 10.1097/00007435-199901000-00005. Sex Transm Dis. 1999. PMID: 9918320
-
The risk of transmission of genital Chlamydia trachomatis infection is less than that of genital Neisseria gonorrhoeae infection.Sex Transm Dis. 1980 Jan-Mar;7(1):6-10. doi: 10.1097/00007435-198001000-00002. Sex Transm Dis. 1980. PMID: 6771879
-
Diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis infections using antigen detection methods.Diagn Microbiol Infect Dis. 1986 Mar;4(3 Suppl):93S-99S. doi: 10.1016/s0732-8893(86)80047-5. Diagn Microbiol Infect Dis. 1986. PMID: 3084162 Review.
Cited by
-
Engineered Tissue Models to Decode Host-Microbiota Interactions.Adv Sci (Weinh). 2025 Jun;12(23):e2417687. doi: 10.1002/advs.202417687. Epub 2025 May 14. Adv Sci (Weinh). 2025. PMID: 40364768 Free PMC article. Review.
-
A straightforward cell culture insert model to incorporate biochemical and biophysical stromal properties into transplacental transport studies.bioRxiv [Preprint]. 2024 Apr 25:2024.04.19.590317. doi: 10.1101/2024.04.19.590317. bioRxiv. 2024. Update in: Placenta. 2025 Jun 13;166:54-61. doi: 10.1016/j.placenta.2024.09.001. PMID: 38712271 Free PMC article. Updated. Preprint.
-
A straightforward cell culture insert model to incorporate biochemical and biophysical stromal properties into transplacental transport studies.Placenta. 2025 Jun 13;166:54-61. doi: 10.1016/j.placenta.2024.09.001. Epub 2024 Sep 5. Placenta. 2025. PMID: 39266436
-
Study Models for Chlamydia trachomatis Infection of the Female Reproductive Tract.Microorganisms. 2025 Feb 28;13(3):553. doi: 10.3390/microorganisms13030553. Microorganisms. 2025. PMID: 40142446 Free PMC article. Review.
-
The human vaginal microbiota: from clinical medicine to models to mechanisms.Curr Opin Microbiol. 2024 Feb;77:102422. doi: 10.1016/j.mib.2023.102422. Epub 2024 Jan 11. Curr Opin Microbiol. 2024. PMID: 38215548 Free PMC article. Review.
References
-
- Abbott A. Cell culture: biology's new dimension. Nature. 2003;424:870–2. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

