Yeast osmoregulation - glycerol still in pole position
- PMID: 35927716
- PMCID: PMC9428294
- DOI: 10.1093/femsyr/foac035
Yeast osmoregulation - glycerol still in pole position
Abstract
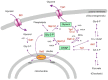
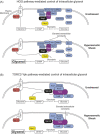
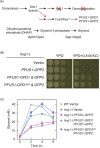
In response to osmotic dehydration cells sense, signal, alter gene expression, and metabolically counterbalance osmotic differences. The main compatible solute/osmolyte that accumulates in yeast cells is glycerol, which is produced from the glycolytic intermediate dihydroxyacetone phosphate. This review covers recent advancements in understanding mechanisms involved in sensing, signaling, cell-cycle delays, transcriptional responses as well as post-translational modifications on key proteins in osmoregulation. The protein kinase Hog1 is a key-player in many of these events, however, there is also a growing body of evidence for important Hog1-independent mechanisms playing vital roles. Several missing links in our understanding of osmoregulation will be discussed and future avenues for research proposed. The review highlights that this rather simple experimental system-salt/sorbitol and yeast-has developed into an enormously potent model system unravelling important fundamental aspects in biology.
Keywords: cell cycle delay; gene expression; osmo-sensing; osmo-signaling; osmoregulation; yeast.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Figures


Similar articles
-
Yeast osmoregulation.Methods Enzymol. 2007;428:29-45. doi: 10.1016/S0076-6879(07)28002-4. Methods Enzymol. 2007. PMID: 17875410 Review.
-
Osmoregulation in Saccharomyces cerevisiae via mechanisms other than the high-osmolarity glycerol pathway.Microbiology (Reading). 2016 Sep;162(9):1511-1526. doi: 10.1099/mic.0.000360. Epub 2016 Aug 23. Microbiology (Reading). 2016. PMID: 27557593 Review.
-
A systems-level analysis of perfect adaptation in yeast osmoregulation.Cell. 2009 Jul 10;138(1):160-71. doi: 10.1016/j.cell.2009.04.047. Cell. 2009. PMID: 19596242 Free PMC article.
-
Osmoresponsive proteins and functional assessment strategies in Saccharomyces cerevisiae.Electrophoresis. 1997 Aug;18(8):1429-40. doi: 10.1002/elps.1150180818. Electrophoresis. 1997. PMID: 9298657 Review.
-
Osmotic adaptation in yeast--control of the yeast osmolyte system.Int Rev Cytol. 2002;215:149-87. doi: 10.1016/s0074-7696(02)15008-x. Int Rev Cytol. 2002. PMID: 11952227 Review.
Cited by
-
Gene-by-environment interactions influence the fitness cost of gene copy-number variation in yeast.G3 (Bethesda). 2023 Sep 30;13(10):jkad159. doi: 10.1093/g3journal/jkad159. G3 (Bethesda). 2023. PMID: 37481264 Free PMC article.
-
Osmotically Activated Anion Current of Phycomyces Blakesleeanus-Filamentous Fungi Counterpart to Vertebrate Volume Regulated Anion Current.J Fungi (Basel). 2023 May 31;9(6):637. doi: 10.3390/jof9060637. J Fungi (Basel). 2023. PMID: 37367573 Free PMC article.
-
Frog-killing chytrid fungi deploy different strategies to regulate intracellular pressure in cell types that have or lack a cell wall.bioRxiv [Preprint]. 2025 May 14:2025.05.13.653819. doi: 10.1101/2025.05.13.653819. bioRxiv. 2025. PMID: 40462924 Free PMC article. Preprint.
-
The phenomenon of anhydrobiosis-structural and functional changes in yeast cells.Appl Microbiol Biotechnol. 2025 Jun 25;109(1):152. doi: 10.1007/s00253-025-13539-6. Appl Microbiol Biotechnol. 2025. PMID: 40560253 Free PMC article. Review.
-
Response mechanism of ethanol-tolerant Saccharomyces cerevisiae strain ES-42 to increased ethanol during continuous ethanol fermentation.Microb Cell Fact. 2025 Jan 30;24(1):33. doi: 10.1186/s12934-025-02663-7. Microb Cell Fact. 2025. PMID: 39885572 Free PMC article.
References
-
- Ahmadpour D, Geijer C, Tamas MJet al. . Yeast reveals unexpected roles and regulatory features of aquaporins and aquaglyceroporins. Biochim Biophys Acta. 2014;1840:1482–91. - PubMed
-
- Akhtar N, Blomberg A, Adler L. Osmoregulation and protein expression in a pbs2∆ mutant of Saccharomyces cerevisiae during adaptation to hypersaline stress. FEBS Lett. 1997;403:173–80. - PubMed
-
- Albertyn J, Hohmann S, Thevelein JMet al. . GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomycescerevisiae, and its expression is regulated by the high osmolarity glycerol response pathway. Mol Cell Biol. 1994;14:4135–44. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

