Randomised controlled trial comparing the clinical and cost-effectiveness of various washout policies versus no washout policy in preventing catheter associated complications in adults living with long-term catheters: study protocol for the CATHETER II study
- PMID: 35927733
- PMCID: PMC9351274
- DOI: 10.1186/s13063-022-06577-2
Randomised controlled trial comparing the clinical and cost-effectiveness of various washout policies versus no washout policy in preventing catheter associated complications in adults living with long-term catheters: study protocol for the CATHETER II study
Abstract
Background: Various washout policies are widely used in adults living with long-term catheters (LTC). There is currently insufficient evidence on the benefits and potential harms of prophylactic LTC washout policies in the prevention of blockages and other LTC-related adverse events, such as urinary tract infections. CATHETER II tests the hypothesis that weekly prophylactic LTC washouts (normal saline or citric acid) in addition to standard LTC care reduce the incidence of catheter blockage requiring intervention compared to standard LTC care only in adults living with LTC.
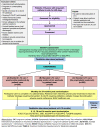
Methods: CATHETER II is a pragmatic three-arm open multi-centre superiority randomised controlled trial with an internal pilot, economic analysis, and embedded qualitative study. Eligible participants are adults aged ≥ 18 years, who have had a LTC in use for ≥ 28 days, have no plans to discontinue the use of the catheter, are able to undertake the catheter washouts, and complete trial documentation or have a carer able to help them. Participants are identified from general practitioner practices, secondary/tertiary care, community healthcare, care homes, and via public advertising strategies. Participants are randomised 1:1:1 to receive a weekly saline (0.9%) washout in addition to standard LTC care, a weekly citric acid (3.23%) washout in addition to standard LTC care or standard LTC care only. Participants and/or carers will receive training to administer the washouts. Patient-reported outcomes are collected at baseline and for 24 months post-randomisation. The primary clinical outcome is catheter blockage requiring intervention up to 24 months post-randomisation expressed per 1000 catheter days. Secondary outcomes include symptomatic catheter-associated urinary tract infection requiring antibiotics, catheter change, adverse events, NHS/ healthcare use, and impact on quality of life.
Discussion: This study will guide treatment decision-making and clinical practice guidelines regarding the effectiveness of various prophylactic catheter washout policies in men and women living with LTC. This research has received ethical approval from Wales Research Ethics Committee 6 (19/WA/0015).
Trial registration: ISRCTN ISRCTN17116445 . Registered prospectively on 06 November 2019.
Keywords: Catheter blockage; Catheter maintenance solutions; Catheter washout solutions; Indwelling catheter; Long-term catheter; Symptomatic catheter-associated urinary tract infection.
© 2022. The Author(s).
Conflict of interest statement
The study is funded by the NIHR Health Technology Assessment. B. Braun Medical AG donated the supply of washout solutions for use in the CATHETER II study. B. Braun Medical AG had no role in the design of the study, collection of data and the writing of this paper and will not have a role in future collection and analysis and interpretation of the data. B.Braun Medical AG may comment on future manuscripts prior to submission, and these shall be considered so long as they do not compromise the scientific nature or neutrality of the publication. The authors declare that they have no competing interests.
Figures
Similar articles
-
CATHETER II: a randomised controlled trial comparing the clinical effectiveness of various washout policies versus no washout policy in preventing catheter-associated complications in adults living with long-term catheters.BMJ Open. 2024 Dec 2;14(12):e087203. doi: 10.1136/bmjopen-2024-087203. BMJ Open. 2024. PMID: 39622574 Free PMC article. Clinical Trial.
-
Patient and healthcare professionals' perception of weekly prophylactic catheter washout in adults living with long-term catheters: qualitative study of the CATHETER II trial.BMJ Open. 2025 Apr 7;15(4):e087206. doi: 10.1136/bmjopen-2024-087206. BMJ Open. 2025. PMID: 40194879 Free PMC article. Clinical Trial.
-
Washout policies in long-term indwelling urinary catheterization in adults: a short version cochrane review.Neurourol Urodyn. 2011 Sep;30(7):1208-12. doi: 10.1002/nau.21063. Epub 2011 May 11. Neurourol Urodyn. 2011. PMID: 21563211 Review.
-
Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial).Health Technol Assess. 2012 Nov;16(47):1-197. doi: 10.3310/hta16470. Health Technol Assess. 2012. PMID: 23199586 Clinical Trial.
-
Types of urethral catheters for management of short-term voiding problems in hospitalized adults: a short version Cochrane review.Neurourol Urodyn. 2008;27(8):738-46. doi: 10.1002/nau.20645. Neurourol Urodyn. 2008. PMID: 18951451 Review.
Cited by
-
A case report of three people experiencing intractable autonomic dysreflexia following instillation of Uro-Tainer® Polyhexanide 0.02.Spinal Cord Ser Cases. 2024 Apr 5;10(1):17. doi: 10.1038/s41394-024-00626-5. Spinal Cord Ser Cases. 2024. PMID: 38580624 Free PMC article.
-
CATHETER II: a randomised controlled trial comparing the clinical effectiveness of various washout policies versus no washout policy in preventing catheter-associated complications in adults living with long-term catheters.BMJ Open. 2024 Dec 2;14(12):e087203. doi: 10.1136/bmjopen-2024-087203. BMJ Open. 2024. PMID: 39622574 Free PMC article. Clinical Trial.
-
Patient and healthcare professionals' perception of weekly prophylactic catheter washout in adults living with long-term catheters: qualitative study of the CATHETER II trial.BMJ Open. 2025 Apr 7;15(4):e087206. doi: 10.1136/bmjopen-2024-087206. BMJ Open. 2025. PMID: 40194879 Free PMC article. Clinical Trial.
References
-
- National Institute for Health and Clinical Excellence (NICE). Healthcare-associated infections: prevention and control in primary and community care. 2017; Available at: www.nice.org.uk/guidance/cg139. Accessed 31 Mar 2021. - PubMed
-
- National Health Service. Urinary catheters. 2020; Available at: https://www.nhs.uk/conditions/urinary-catheters/. Accessed 11 Apr 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical