Dominant changes in the breast muscle lipid profiles of broiler chickens with wooden breast syndrome revealed by lipidomics analyses
- PMID: 35927736
- PMCID: PMC9354336
- DOI: 10.1186/s40104-022-00743-x
Dominant changes in the breast muscle lipid profiles of broiler chickens with wooden breast syndrome revealed by lipidomics analyses
Abstract
Background: Chicken is the most consumed meat worldwide and the industry has been facing challenging myopathies. Wooden breast (WB), which is often accompanied by white striping (WS), is a serious myopathy adversely affecting meat quality of breast muscles. The underlying lipid metabolic mechanism of WB affected broilers is not fully understood.
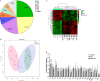
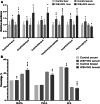
Results: A total of 150 chickens of a white-feathered, fast-growing pure line were raised and used for the selection of WB, WB + WS and control chickens. The lipids of the breast muscle, liver, and serum from different chickens were extracted and measured using ultra performance liquid chromatography (UPLC) plus Q-Exactive Orbitrap tandem mass spectrometry. In the breast, 560 lipid molecules were identified. Compared to controls, 225/225 of 560 lipid molecules (40.2%) were identified with differential abundance (DA), including 92/100 significantly increased neutral lipids and 107/98 decreased phospholipids in the WB/WB + WS groups, respectively. The content of monounsaturated fatty acids (MUFA) was significantly higher, and the polyunsaturated fatty acids (PUFA) and saturated fatty acids (SFA) were significantly lower in the affected breasts. In the liver, 434 lipid molecules were identified, and 39/61 DA lipid molecules (6.7%/14.1%) were detected in the WB and WB + WS groups, respectively. In the serum, a total of 529 lipid molecules were identified and 4/44 DA lipid molecules (0.8%/8.3%) were detected in WB and WB + WS group, respectively. Compared to controls, the content of MUFAs in the serum and breast of the WB + WS group were both significantly increased, and the content of SFAs in two tissues were both significantly decreased. Only five lipid molecules were consistently increased in both liver and serum in WB + WS group.
Conclusions: We have found for the first time that the dominant lipid profile alterations occurred in the affected breast muscle. The relative abundance of 40.2% of lipid molecules were changed and is characteristic of increased neutral lipids and decreased phospholipids in the affected breasts. Minor changes of lipid profiles in the liver and serum of the affected groups were founded. Comprehensive analysis of body lipid metabolism indicated that the abnormal lipid profile of WB breast may be independent of the liver metabolism.
Keywords: Broiler chicken; Lipid profile; Liver; Serum; Wooden breast.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Fatty Acid Profile, Volatile Organic Compound, and Physical Parameter Changes in Chicken Breast Meat Affected by Wooden Breast and White Striping Myopathies.Animals (Basel). 2023 Oct 7;13(19):3136. doi: 10.3390/ani13193136. Animals (Basel). 2023. PMID: 37835742 Free PMC article.
-
Occurrence of wooden breast and white striping in Brazilian slaughtering plants and use of near-infrared spectroscopy and multivariate analysis to identify affected chicken breasts.J Food Sci. 2020 Oct;85(10):3102-3112. doi: 10.1111/1750-3841.15465. Epub 2020 Sep 29. J Food Sci. 2020. PMID: 32996140
-
Effect of breast myopathies on quality and microbial shelf life of broiler meat.Poult Sci. 2019 Jun 1;98(6):2641-2651. doi: 10.3382/ps/pez001. Poult Sci. 2019. PMID: 30668837
-
White striping and woody breast myopathies in the modern poultry industry: a review.Poult Sci. 2016 Nov 1;95(11):2724-2733. doi: 10.3382/ps/pew216. Epub 2016 Jul 21. Poult Sci. 2016. PMID: 27450434 Review.
-
The Incidence of Muscle Abnormalities in Broiler Breast Meat - A Review.Korean J Food Sci Anim Resour. 2018 Oct;38(5):835-850. doi: 10.5851/kosfa.2018.e2. Epub 2018 Oct 31. Korean J Food Sci Anim Resour. 2018. PMID: 30479493 Free PMC article. Review.
Cited by
-
Comparative metabolomic analysis of spaghetti meat and wooden breast in broiler chickens: unveiling similarities and dissimilarities.Front Physiol. 2024 Oct 9;15:1456664. doi: 10.3389/fphys.2024.1456664. eCollection 2024. Front Physiol. 2024. PMID: 39444756 Free PMC article.
-
Metabolomic Analysis of Wooden Breast Myopathy Shows a Disturbed Lipid Metabolism.Metabolites. 2022 Dec 22;13(1):20. doi: 10.3390/metabo13010020. Metabolites. 2022. PMID: 36676945 Free PMC article.
-
The hypoxia-inducible factor 1 pathway plays a critical role in the development of breast muscle myopathies in broiler chickens: a comprehensive review.Front Physiol. 2023 Aug 31;14:1260987. doi: 10.3389/fphys.2023.1260987. eCollection 2023. Front Physiol. 2023. PMID: 37719466 Free PMC article. Review.
-
Molecular Regulation of Differential Lipid Molecule Accumulation in the Intramuscular Fat and Abdominal Fat of Chickens.Genes (Basel). 2023 Jul 17;14(7):1457. doi: 10.3390/genes14071457. Genes (Basel). 2023. PMID: 37510361 Free PMC article.
-
Monitoring physiological processes of fast-growing broilers during the whole life cycle: Changes of redox-homeostasis effected to trassulfuration pathway predicting the development of non-alcoholic fatty liver disease.PLoS One. 2023 Aug 17;18(8):e0290310. doi: 10.1371/journal.pone.0290310. eCollection 2023. PLoS One. 2023. PMID: 37590293 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

