Purification, Partial Characterization, and Evaluation of the Antiulcer Activity of Calotropis procera Leaf Lectin
- PMID: 35927810
- PMCID: PMC9896378
- DOI: 10.2174/0929866529666220803162457
Purification, Partial Characterization, and Evaluation of the Antiulcer Activity of Calotropis procera Leaf Lectin
Abstract
Background: Lectins are proteins with therapeutic and diagnostic potential that can be applied in battling various ailments.
Aim and objective: This study was designed to purify and characterize the hemagglutinating activity derived from the leaves of Calotropis procera and its possible role in protecting the stomach against ethanol-induced lesions.
Methods: The Calotropis procera leaf lectin (ProLec), was isolated by homogenization of the defatted leaf powder in Phosphate-Buffered Saline (PBS) and purified by affinity chromatography on Sephadex G-100. The lectin was eluted from the affinity column by 3% acetic acid and was physicochemically characterized. In a dose-dependent manner, ProLec was administered to rats with ethanol-induced ulcers, and biochemical, histopathological, and toxicological examinations were performed.
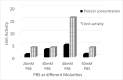
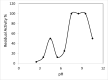
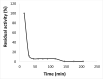
Results: ProLec is a heterodimer of 75 and 68 kDa. It agglutinated all human RBCs, whereas it showed weak interaction with animal erythrocytes. The protein was optimally active at 25 °C and was labile above this temperature. ProLec exhibited two pH optima and was a metalloprotein requiring Ca, Mn, and Ni. It contains 1.6% tryptophan residues of which about 1% is exposed and critical for lectin activity. The lectin exhibited a potent gastroprotective effect against ethanolinduced gastric lesions with no apparent toxicity to both kidneys and liver. Examination of the pH of the gastric juice of lectin-treated animals indicated a possible role of lectin in maintaining stomach acidity within the normal ranges compared to the gastric juice pH of animals that received ethanol only.
Conclusion: These results may suggest that ProLec could conceivably be a good future drug for the treatment of gastric ulcers, however, extensive immunological and toxicological research remains to be done.
Keywords: Calotropis procera; gastroprotective; lectin; medicinal plant; purification; ulcer.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures











Similar articles
-
Protective effect of Calotropis procera latex extracts on experimentally induced gastric ulcers in rat.J Ethnopharmacol. 2010 Feb 3;127(2):440-4. doi: 10.1016/j.jep.2009.10.016. Epub 2009 Oct 21. J Ethnopharmacol. 2010. PMID: 19853030
-
Chemometric profile & antimicrobial activities of leaf extract of Calotropis procera and Calotropis gigantea.Nat Prod Res. 2017 Aug;31(16):1954-1957. doi: 10.1080/14786419.2016.1266349. Epub 2016 Dec 9. Nat Prod Res. 2017. PMID: 27936921
-
Antiulcer mechanisms of Vernonia condensata Baker: A medicinal plant used in the treatment of gastritis and gastric ulcer.J Ethnopharmacol. 2016 May 26;184:196-207. doi: 10.1016/j.jep.2016.02.049. Epub 2016 Mar 5. J Ethnopharmacol. 2016. PMID: 26956376
-
Cochlospermum regium (Mart. ex Schrank) Pilg.: Evaluation of chemical profile, gastroprotective activity and mechanism of action of hydroethanolic extract of its xylopodium in acute and chronic experimental models.J Ethnopharmacol. 2019 Apr 6;233:101-114. doi: 10.1016/j.jep.2019.01.002. Epub 2019 Jan 3. J Ethnopharmacol. 2019. PMID: 30611907
-
Calotropis procera and the Pharmacological Properties of Its Aqueous Leaf Extract: A Review.Cureus. 2024 May 15;16(5):e60354. doi: 10.7759/cureus.60354. eCollection 2024 May. Cureus. 2024. PMID: 38883127 Free PMC article. Review.
Cited by
-
From inflammation to immune regulation: The dual nature of dietary lectins in health and disease.Heliyon. 2024 Oct 18;10(20):e39471. doi: 10.1016/j.heliyon.2024.e39471. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39502251 Free PMC article. Review.
-
Biological activity and characterization of leaf and seed lectins from Terminalia brownii: Insights into their analgesic and antiulcer properties.Heliyon. 2024 Oct 18;10(20):e39351. doi: 10.1016/j.heliyon.2024.e39351. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39498066 Free PMC article.
References
-
- Barbosa P.P.S., De Araújo F.N., De Almeida J.M., Gadelha T.S. Leguminosae lectins as biological tools in medical research: A review. Braz. Arch. Biol. Technol. 2021;64:e21200170. doi: 10.1590/1678-4324-2021200170. - DOI
-
- Sharon N., Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. - PubMed
-
- Cummings R.D. Use of lectins in analysis of glycoconjugates. Methods in enzymology. USA: Academic Press; 1994. pp. 66–86. - PubMed
-
- Osman M.E.M., Konozy E.H.E. Insight into Erythrina lectins: Properties, structure and proposed physiological significance. Open Bioactive Compd. J. 2020;9:57–71.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

