A Phytochrome B-PIF4-MYC2/MYC4 module inhibits secondary cell wall thickening in response to shaded light
- PMID: 35927944
- PMCID: PMC9700123
- DOI: 10.1016/j.xplc.2022.100416
A Phytochrome B-PIF4-MYC2/MYC4 module inhibits secondary cell wall thickening in response to shaded light
Abstract
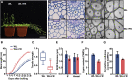
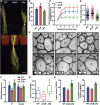
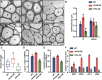
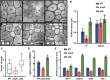
Secondary cell walls (SCWs) in stem cells provide mechanical strength and structural support for growth. SCW thickening varies under different light conditions. Our previous study revealed that blue light enhances SCW thickening through the redundant function of MYC2 and MYC4 directed by CRYPTOCHROME1 (CRY1) signaling in fiber cells of the Arabidopsis inflorescence stem. In this study, we find that the Arabidopsis PHYTOCHROME B mutant phyB displays thinner SCWs in stem fibers, but thicker SCWs are deposited in the PHYTOCHROME INTERACTING FACTOR (PIF) quadruple mutant pif1pif3pif4pif5 (pifq). The shaded light condition with a low ratio of red to far-red light inhibits stem SCW thickening. PIF4 interacts with MYC2 and MYC4 to affect their localization in nuclei, and this interaction results in inhibition of the MYCs' transactivation activity on the NST1 promoter. Genetic evidence shows that regulation of SCW thickening by PIFs is dependent on MYC2/MYC4 function. Together, the results of this study reveal a PHYB-PIF4-MYC2/MYC4 module that inhibits SCW thickening in fiber cells of the Arabidopsis stem.
Keywords: MYC2; far-red light; fiber cell; secondary cell wall; xylem.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Blue Light Regulates Secondary Cell Wall Thickening via MYC2/MYC4 Activation of the NST1-Directed Transcriptional Network in Arabidopsis.Plant Cell. 2018 Oct;30(10):2512-2528. doi: 10.1105/tpc.18.00315. Epub 2018 Sep 21. Plant Cell. 2018. PMID: 30242037 Free PMC article.
-
The molecular mechanism of transforming red light signal to (E)-β-caryophyllene biosynthesis in Arabidopsis.Physiol Plant. 2025 Jan-Feb;177(1):e70065. doi: 10.1111/ppl.70065. Physiol Plant. 2025. PMID: 39835494
-
Assessing the Function of CBF1 in Modulating the Interaction Between Phytochrome B and PIF4.Methods Mol Biol. 2024;2795:183-194. doi: 10.1007/978-1-0716-3814-9_18. Methods Mol Biol. 2024. PMID: 38594539
-
The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels.Plant Cell. 2008 Feb;20(2):337-52. doi: 10.1105/tpc.107.052142. Epub 2008 Feb 5. Plant Cell. 2008. PMID: 18252845 Free PMC article.
-
Phytochrome-interacting factor from Arabidopsis to liverwort.Curr Opin Plant Biol. 2017 Feb;35:54-60. doi: 10.1016/j.pbi.2016.11.004. Epub 2016 Nov 19. Curr Opin Plant Biol. 2017. PMID: 27875778 Review.
Cited by
-
Genome-wide identification of ZmMYC2 binding sites and target genes in maize.BMC Genomics. 2024 Apr 23;25(1):397. doi: 10.1186/s12864-024-10297-z. BMC Genomics. 2024. PMID: 38654166 Free PMC article.
-
Genome-Wide Identification and Expression Profiling of Velvet Complex Transcription Factors in Populus alba × Populus glandulosa.Int J Mol Sci. 2024 Mar 31;25(7):3926. doi: 10.3390/ijms25073926. Int J Mol Sci. 2024. PMID: 38612736 Free PMC article.
-
The SBP-box transcription factor PlSPL2 negatively regulates stem development in herbaceous peony.Plant Cell Rep. 2024 Nov 8;43(12):275. doi: 10.1007/s00299-024-03355-z. Plant Cell Rep. 2024. PMID: 39511032
-
Genome-Wide Identification and Expression Analysis of Dendrocalamus farinosus CCoAOMT Gene Family and the Role of DfCCoAOMT14 Involved in Lignin Synthesis.Int J Mol Sci. 2023 May 18;24(10):8965. doi: 10.3390/ijms24108965. Int J Mol Sci. 2023. PMID: 37240316 Free PMC article.
-
Endogenous and environmental signals in regulating vascular development and secondary growth.Front Plant Sci. 2024 Apr 2;15:1369241. doi: 10.3389/fpls.2024.1369241. eCollection 2024. Front Plant Sci. 2024. PMID: 38628366 Free PMC article. No abstract available.
References
-
- Bauer D., Viczián A., Kircher S., Nobis T., Nitschke R., Kunkel T., Panigrahi K.C.S., Adám E., Fejes E., Schäfer E., et al. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell. 2004;16:1433–1445. doi: 10.1105/tpc.021568. - DOI - PMC - PubMed
-
- Chen R., Jiang H., Li L., Zhai Q., Qi L., Zhou W., Liu X., Li H., Zheng W., Sun J., et al. The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell. 2012;24:2898–2916. doi: 10.1105/tpc.112.098277. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

