A Century Searching for the Neurons Necessary for Wakefulness
- PMID: 35928009
- PMCID: PMC9344068
- DOI: 10.3389/fnins.2022.930514
A Century Searching for the Neurons Necessary for Wakefulness
Abstract
Wakefulness is necessary for consciousness, and impaired wakefulness is a symptom of many diseases. The neural circuits that maintain wakefulness remain incompletely understood, as do the mechanisms of impaired consciousness in many patients. In contrast to the influential concept of a diffuse "reticular activating system," the past century of neuroscience research has identified a focal region of the upper brainstem that, when damaged, causes coma. This region contains diverse neuronal populations with different axonal projections, neurotransmitters, and genetic identities. Activating some of these populations promotes wakefulness, but it remains unclear which specific neurons are necessary for sustaining consciousness. In parallel, pharmacological evidence has indicated a role for special neurotransmitters, including hypocretin/orexin, histamine, norepinephrine, serotonin, dopamine, adenosine and acetylcholine. However, genetically targeted experiments have indicated that none of these neurotransmitters or the neurons producing them are individually necessary for maintaining wakefulness. In this review, we emphasize the need to determine the specific subset of brainstem neurons necessary for maintaining arousal. Accomplishing this will enable more precise mapping of wakefulness circuitry, which will be useful in developing therapies for patients with coma and other disorders of arousal.
Keywords: arousal; ascending reticular activating system; brainstem; coma; wakefulness.
Copyright © 2022 Grady, Boes and Geerling.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
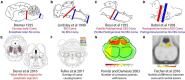
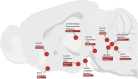
Similar articles
-
[Functional pathophysiology of consciousness].Neuropsychiatr. 2009;23(2):115-33. Neuropsychiatr. 2009. PMID: 19573504 Review. German.
-
Orexin/Hypocretin and Organizing Principles for a Diversity of Wake-Promoting Neurons in the Brain.Curr Top Behav Neurosci. 2017;33:51-74. doi: 10.1007/7854_2016_45. Curr Top Behav Neurosci. 2017. PMID: 27830577 Free PMC article. Review.
-
[Major neurotransmitters involved in the regulation of sleep-wake cycle].Rev Invest Clin. 2012 Mar-Apr;64(2):182-91. Rev Invest Clin. 2012. PMID: 22991780 Review. Spanish.
-
Cellular stress and AMPK links metformin and diverse compounds with accelerated emergence from anesthesia and potential recovery from disorders of consciousness.Med Hypotheses. 2019 Mar;124:42-52. doi: 10.1016/j.mehy.2019.01.014. Epub 2019 Jan 22. Med Hypotheses. 2019. PMID: 30798915
-
Underlying brain mechanisms that regulate sleep-wakefulness cycles.Int Rev Neurobiol. 2010;93:1-21. doi: 10.1016/S0074-7742(10)93001-8. Int Rev Neurobiol. 2010. PMID: 20969999 Review.
Cited by
-
How to predict the outcome of primary brainstem hemorrhage: Six-year results of a single-center retrospective analysis.Medicine (Baltimore). 2023 Sep 15;102(37):e35131. doi: 10.1097/MD.0000000000035131. Medicine (Baltimore). 2023. PMID: 37713883 Free PMC article.
-
Lethal Interactions of neuronal networks in epilepsy mediated by both synaptic and volume transmission indicate approaches to prevention.Prog Neurobiol. 2025 Jun;249:102770. doi: 10.1016/j.pneurobio.2025.102770. Epub 2025 Apr 19. Prog Neurobiol. 2025. PMID: 40258456 Review.
-
Can Pure Thalamic Strokes Lead to Severe Impairment of Arousal?Eur J Neurol. 2025 Jun;32(6):e70106. doi: 10.1111/ene.70106. Eur J Neurol. 2025. PMID: 40433835 Free PMC article.
-
Molecular and cellular targets of GABAergic anesthetics in the mesopontine tegmentum that enable pain-free surgery.Pain. 2024 Dec 30;166(7):1549-1564. doi: 10.1097/j.pain.0000000000003504. Pain. 2024. PMID: 39792547 Free PMC article.
-
Parabrachial Foxp2-expressing neurons are necessary for sustaining core body temperature in the cold.iScience. 2025 Jun 28;28(8):112764. doi: 10.1016/j.isci.2025.112764. eCollection 2025 Aug 15. iScience. 2025. PMID: 40703448 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

