Hypothalamus-Muscle Parallel Induction of Metabolic Pathways Following Physical Exercise
- PMID: 35928013
- PMCID: PMC9344923
- DOI: 10.3389/fnins.2022.897005
Hypothalamus-Muscle Parallel Induction of Metabolic Pathways Following Physical Exercise
Abstract
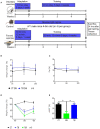
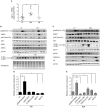
The modern lifestyle requires less physical activity and skills during our daily routine, leading to multiple pathologies related to physical disabilities and energy accessibility. Thus, exploring the mechanisms underlying the metabolic regulation of exercise is crucial. Here, we characterized the effect of forced and voluntary endurance exercises on three key metabolic signaling pathways, sirtuins, AMPK, and mTOR, across several metabolic tissues in mice: brain, muscles, and liver. Both voluntary and forced exercises induced AMPK with higher intensity in the first. The comparison between those metabolic tissues revealed that the hypothalamus and the hippocampus, two brain parts, showed different metabolic signaling activities. Strikingly, despite the major differences in the physiology of muscles and hypothalamic tissues, the hypothalamus replicates the metabolic response of the muscle in response to physical exercise. Specifically, muscles and hypothalamic tissues showed an increase and a decrease in AMPK and mTOR signaling, respectively. Overall, this study reveals new insight into the relation between the hypothalamus and muscles, which enhances the coordination within the muscle-brain axis and potentially improves the systemic response to physical activity performance and delaying health inactivity disorders.
Keywords: exercise; hippocampus; hypothalamus; metabolic pathways; muscle.
Copyright © 2022 Katz, Gonen, Shahar, Roichman, Lerrer and Cohen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Hypothalamus-skeletal muscle crosstalk during exercise and its role in metabolism modulation.Biochem Pharmacol. 2021 Aug;190:114640. doi: 10.1016/j.bcp.2021.114640. Epub 2021 Jun 1. Biochem Pharmacol. 2021. PMID: 34087244 Review.
-
Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior.Behav Brain Res. 2017 Apr 14;323:56-67. doi: 10.1016/j.bbr.2017.01.033. Epub 2017 Jan 24. Behav Brain Res. 2017. PMID: 28130174 Free PMC article.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Exercise enhances insulin and leptin signaling in the cerebral cortex and hypothalamus during dexamethasone-induced stress in diabetic rats.Neuroendocrinology. 2005;82(5-6):282-93. doi: 10.1159/000093127. Epub 2006 May 4. Neuroendocrinology. 2005. PMID: 16721034
-
An "enigmatic" L-carnosine (β-alanyl-L-histidine)? Cell proliferative activity as a fundamental property of a natural dipeptide inherent to traditional antioxidant, anti-aging biological activities: balancing and a hormonally correct agent, novel patented oral therapy dosage formulation for mobility, skeletal muscle power and functional performance, hypothalamic-pituitary- brain relationship in health, aging and stress studies.Recent Pat Drug Deliv Formul. 2015;9(1):1-64. doi: 10.2174/1872211309666141218145408. Recent Pat Drug Deliv Formul. 2015. PMID: 25524476 Review.
Cited by
-
The association of hemopexin, muscle quality, and sarcopenia in Japanese older adults with cognitive impairment: a cross-sectional study.BMC Geriatr. 2025 May 13;25(1):332. doi: 10.1186/s12877-025-05977-8. BMC Geriatr. 2025. PMID: 40361004 Free PMC article.
-
Cell biomechanics on muscle atrophy: from intricate mechanisms to therapeutic frontiers.Ann Med. 2025 Dec;57(1):2540598. doi: 10.1080/07853890.2025.2540598. Epub 2025 Aug 1. Ann Med. 2025. PMID: 40747836 Free PMC article. Review.
-
Exercise Restores Hypothalamic Health in Obesity by Reshaping the Inflammatory Network.Antioxidants (Basel). 2023 Jan 28;12(2):297. doi: 10.3390/antiox12020297. Antioxidants (Basel). 2023. PMID: 36829858 Free PMC article. Review.
-
Muscle-brain crosstalk mediated by exercise-induced myokines - insights from experimental studies.Front Physiol. 2024 Dec 2;15:1488375. doi: 10.3389/fphys.2024.1488375. eCollection 2024. Front Physiol. 2024. PMID: 39687518 Free PMC article. Review.
References
-
- Aguiar A. S., Castro A., Moreira E. L., Glaser V., Santos A. R. S., Tasca C. I., et al. (2011). Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: Involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech. Ageing Dev. 132, 560–567. 10.1016/j.mad.2011.09.005 - DOI - PubMed
-
- Azimi M., Gharakhanou R., Naghdi N., Khodadadi D., Heysieattalab S. (2018). Moderate treadmill exercise ameliorates amyloid-β-induced learning and memory impairment, possibly via increasing AMPK activity and up-regulation of the PGC-1α/FNDC5/BDNF pathway. Peptides. 102, 78–88. 10.1016/j.peptides.2017.12.027 - DOI - PubMed
-
- Berg J. M., Tymoczko J. L., Stryer L. (2002). Each Organ Has a Unique Metabolic Profile-Biochemistry, 5th Edition (New York, NY: W. H. Freeman publishing; ).
LinkOut - more resources
Full Text Sources
Miscellaneous

