Identification of cytochrome c oxidase subunit 4 isoform 1 as a positive regulator of influenza virus replication
- PMID: 35928150
- PMCID: PMC9343726
- DOI: 10.3389/fmicb.2022.862205
Identification of cytochrome c oxidase subunit 4 isoform 1 as a positive regulator of influenza virus replication
Abstract
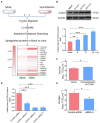
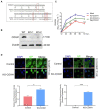
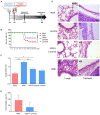
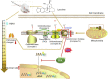
Human infection with highly pathogenic H5N1 influenza virus causes severe respiratory diseases. Currently, the drugs against H5N1 are limited to virus-targeted inhibitors. However, drug resistance caused by these inhibitors is becoming a serious threat to global public health. An alternative strategy to reduce the resistance risk is to develop antiviral drugs targeting host cell proteins. In this study, we demonstrated that cytochrome c oxidase subunit 4 isoform 1 (COX41) of host cell plays an important role in H5N1 infection. Overexpression of COX41 promoted viral replication, which was inhibited by silencing or knockout the expression of COX41 in the host cell. The ribonucleoproteins (RNPs) of H5N1 were retained in the cell nucleus after knockout cellular COX41. Strikingly, inhibition of cellular COX41 by lycorine, a small-molecule compound isolated from Amaryllidaceae plants, reduced the levels of COX41-induced ROS and phosphorylation of extracellular signal-regulated kinase (ERK) in cells, thus resulting in the blockage of nuclear export of vRNP and inhibition of viral replication. In H5N1-infected mice that were treated with lycorine, we observed a reduction of viral titers and inhibition of pathological changes in the lung and trachea tissues. Importantly, no resistant virus was generated after culturing the virus with the continuous treatment of lycorine. Collectively, these findings suggest that COX41 is a positive regulator of H5N1 replication and might serve as an alternative target for anti-influenza drug development.
Keywords: COX41; anti-influenza; highly pathogenic H5N1 influenza virus; lycorine; viral ribonucleoproteins export.
Copyright © 2022 He, Huang, Li, Li, Zhao, Li, Ye, Qi, Tang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

