Butyrate Treatment of DSS-Induced Ulcerative Colitis Affects the Hepatic Drug Metabolism in Mice
- PMID: 35928257
- PMCID: PMC9343805
- DOI: 10.3389/fphar.2022.936013
Butyrate Treatment of DSS-Induced Ulcerative Colitis Affects the Hepatic Drug Metabolism in Mice
Abstract
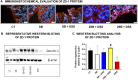
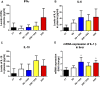
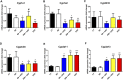
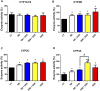
The development of inflammatory bowel disease (IBD) is associated with alterations in the gut microbiota. There is currently no universal treatment for this disease, thus emphasizing the importance of developing innovative therapeutic approaches. Gut microbiome-derived metabolite butyrate with its well-known anti-inflammatory effect in the gut is a promising candidate. Due to increased intestinal permeability during IBD, butyrate may also reach the liver and influence liver physiology, including hepatic drug metabolism. To get an insight into this reason, the aim of this study was set to clarify not only the protective effects of the sodium butyrate (SB) administration on colonic inflammation but also the effects of SB on hepatic drug metabolism in experimental colitis induced by dextran sodium sulfate (DSS) in mice. It has been shown here that the butyrate pre-treatment can alleviate gut inflammation and reduce the leakiness of colonic epithelium by restoration of the assembly of tight-junction protein Zonula occludens-1 (ZO-1) in mice with DSS-induced colitis. In this article, butyrate along with inflammation has also been shown to affect the expression and enzyme activity of selected cytochromes P450 (CYPs) in the liver of mice. In this respect, CYP3A enzymes may be very sensitive to gut microbiome-targeted interventions, as significant changes in CYP3A expression and activity in response to DSS-induced colitis and/or butyrate treatment have also been observed. With regard to medications used in IBD and microbiota-targeted therapeutic approaches, it is important to deepen our knowledge of the effect of gut inflammation, and therapeutic interventions were followed concerning the ability of the organism to metabolize drugs. This gut-liver axis, mediated through inflammation as well as microbiome-derived metabolites, may affect the response to IBD therapy.
Keywords: butyrate; cytochromes P450; drug metabolism; gut inflammation; gut–liver axis.
Copyright © 2022 Jourova, Satka, Frybortova, Zapletalova, Anzenbacher, Anzenbacherova, Hermanova, Drabonova, Srutkova, Kozakova and Hudcovic.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Effect of DSS-Induced Ulcerative Colitis and Butyrate on the Cytochrome P450 2A5: Contribution of the Microbiome.Int J Mol Sci. 2022 Oct 1;23(19):11627. doi: 10.3390/ijms231911627. Int J Mol Sci. 2022. PMID: 36232929 Free PMC article.
-
Spinal anesthesia alleviates dextran sodium sulfate-induced colitis by modulating the gut microbiota.World J Gastroenterol. 2022 Mar 28;28(12):1239-1256. doi: 10.3748/wjg.v28.i12.1239. World J Gastroenterol. 2022. PMID: 35431512 Free PMC article.
-
Dextran sulfate sodium-induced colitis and ginseng intervention altered oral pharmacokinetics of cyclosporine A in rats.J Ethnopharmacol. 2021 Jan 30;265:113251. doi: 10.1016/j.jep.2020.113251. Epub 2020 Aug 15. J Ethnopharmacol. 2021. PMID: 32810615
-
Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review.Nutrients. 2023 May 11;15(10):2275. doi: 10.3390/nu15102275. Nutrients. 2023. PMID: 37242159 Free PMC article. Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Priority order of neonatal colonization by a probiotic or pathogenic Escherichia coli strain dictates the host response to experimental colitis.Front Microbiol. 2024 Aug 14;15:1393732. doi: 10.3389/fmicb.2024.1393732. eCollection 2024. Front Microbiol. 2024. PMID: 39206364 Free PMC article.
-
Effect of DSS-Induced Ulcerative Colitis and Butyrate on the Cytochrome P450 2A5: Contribution of the Microbiome.Int J Mol Sci. 2022 Oct 1;23(19):11627. doi: 10.3390/ijms231911627. Int J Mol Sci. 2022. PMID: 36232929 Free PMC article.
-
Milk and Lacticaseibacillus paracasei BL23 effects on intestinal responses in a murine model of colitis.Am J Physiol Gastrointest Liver Physiol. 2024 Jun 1;326(6):G659-G675. doi: 10.1152/ajpgi.00259.2023. Epub 2024 Apr 9. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38591132 Free PMC article.
-
The effect of oral butyrate on colonic short-chain fatty acid transporters and receptors depends on microbial status.Front Pharmacol. 2024 Mar 26;15:1341333. doi: 10.3389/fphar.2024.1341333. eCollection 2024. Front Pharmacol. 2024. PMID: 38595917 Free PMC article.
-
Postbiotics as Mitochondrial Modulators in Inflammatory Bowel Disease: Mechanistic Insights and Therapeutic Potential.Biomolecules. 2025 Jul 1;15(7):954. doi: 10.3390/biom15070954. Biomolecules. 2025. PMID: 40723826 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

