Genetic causes of central precocious puberty
- PMID: 35928377
- PMCID: PMC9297165
- DOI: 10.1297/cpe.2022-0021
Genetic causes of central precocious puberty
Abstract
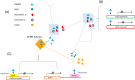
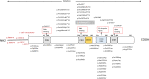
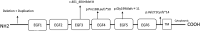
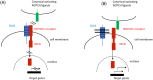
Central precocious puberty (CPP) is a condition in which the hypothalamus-pituitary-gonadal system is activated earlier than the normal developmental stage. The etiology includes organic lesions in the brain; however, in the case of idiopathic diseases, environmental and/or genetic factors are involved in the development of CPP. A genetic abnormality in KISS1R, that encodes the kisspeptin receptor, was first reported in 2008 as a cause of idiopathic CPP. Furthermore, genetic alterations in KISS1, MKRN3, DLK1, and PROKR2 have been reported in idiopathic and/or familial CPP. Of these, MKRN3 has the highest frequency of pathological variants associated with CPP worldwide; but, abnormalities in MKRN3 are rare in patients in East Asia, including Japan. MKRN3 and DLK1 are maternal imprinting genes; thus, CPP develops when a pathological variant is inherited from the father. The mechanism of CPP due to defects in MKRN3 and DLK1 has not been completely clarified, but it is suggested that both may negatively control the progression of puberty. CPP due to such a single gene abnormality is extremely rare, but it is important to understand the mechanisms of puberty and reproduction. A further development in the genetics of CPP is expected in the future.
Keywords: DLK1; MKRN3; genetic factor; precocious puberty.
2022©The Japanese Society for Pediatric Endocrinology.
Figures
Similar articles
-
Novel variants ensued genomic imprinting in familial central precocious puberty.J Endocrinol Invest. 2024 Aug;47(8):2041-2052. doi: 10.1007/s40618-023-02300-3. Epub 2024 Feb 17. J Endocrinol Invest. 2024. PMID: 38367171 Free PMC article.
-
Comprehensive Study on Central Precocious Puberty: Molecular and Clinical Analyses in 90 Patients.J Clin Endocrinol Metab. 2025 Mar 17;110(4):1023-1036. doi: 10.1210/clinem/dgae666. J Clin Endocrinol Metab. 2025. PMID: 39324648
-
Pathogenic and Low-Frequency Variants in Children With Central Precocious Puberty.Front Endocrinol (Lausanne). 2021 Sep 24;12:745048. doi: 10.3389/fendo.2021.745048. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34630334 Free PMC article.
-
Chinese familial central precocious puberty with hyperuricemia due to recurrent DLK1 mutation: Case report and review of the literature.Mol Genet Genomic Med. 2022 Dec;10(12):e2087. doi: 10.1002/mgg3.2087. Epub 2022 Nov 9. Mol Genet Genomic Med. 2022. PMID: 36353763 Free PMC article. Review.
-
Pioneering studies on monogenic central precocious puberty.Arch Endocrinol Metab. 2019 Aug 22;63(4):438-444. doi: 10.20945/2359-3997000000164. Arch Endocrinol Metab. 2019. PMID: 31460623 Free PMC article. Review.
Cited by
-
A review of the genetics and epigenetics of central precocious puberty.Front Endocrinol (Lausanne). 2022 Dec 2;13:1029137. doi: 10.3389/fendo.2022.1029137. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36531492 Free PMC article. Review.
-
Clinical Management and Therapy of Precocious Puberty in the Sapienza University Pediatrics Hospital of Rome, Italy.Children (Basel). 2023 Oct 10;10(10):1672. doi: 10.3390/children10101672. Children (Basel). 2023. PMID: 37892335 Free PMC article. Review.
-
Central precocious puberty in Prader-Willi syndrome: a narrative review.Front Endocrinol (Lausanne). 2023 May 8;14:1150323. doi: 10.3389/fendo.2023.1150323. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37251677 Free PMC article. Review.
-
Novel variants ensued genomic imprinting in familial central precocious puberty.J Endocrinol Invest. 2024 Aug;47(8):2041-2052. doi: 10.1007/s40618-023-02300-3. Epub 2024 Feb 17. J Endocrinol Invest. 2024. PMID: 38367171 Free PMC article.
-
Genetic variants of G-protein coupled receptors associated with pubertal disorders.Reprod Med Biol. 2023 Apr 27;22(1):e12515. doi: 10.1002/rmb2.12515. eCollection 2023 Jan-Dec. Reprod Med Biol. 2023. PMID: 37122876 Free PMC article. Review.
References
-
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 2001;22: 111–51. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
