Catalytic Oxidative Coupling of Phenols and Related Compounds
- PMID: 35928569
- PMCID: PMC9345132
- DOI: 10.1021/acscatal.2c00318
Catalytic Oxidative Coupling of Phenols and Related Compounds
Abstract
Phenols and their derivatives are the elementary building blocks for several classes of complex molecules that play essential roles in biological systems. Nature has devised methods to selectively couple phenolic compounds, and many efforts have been undertaken by chemists to mimic such coupling processes. A range of mechanisms can be involved and with well-studied catalysts, reaction outcomes in phenol-phenol oxidative coupling reactions can be predicted with a good level of fidelity. However, reactions with catalysts that have not been studied or that do not behave similarly to known catalysts can be hard to predict and control. This Perspective provides an overview of catalytic methods for the oxidative coupling of phenols, focusing on the last 10 years, and summarizes current challenges.
Keywords: biphenols; cross-coupling; dehydrogenative coupling; oxidative coupling; phenol coupling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
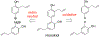
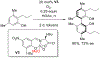
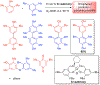

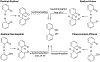
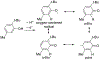
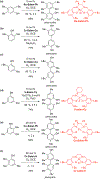
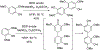
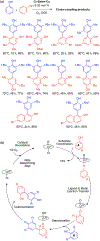

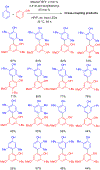
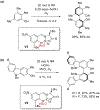
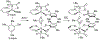


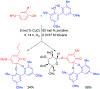
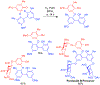

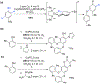
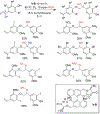
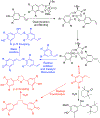
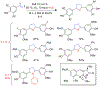
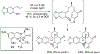
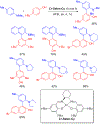
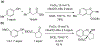
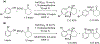

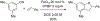
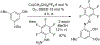
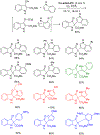
References
-
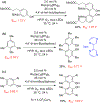
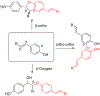
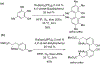
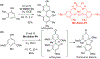
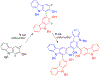
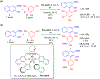
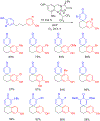
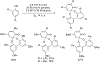
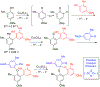
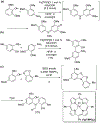
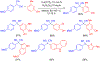
- Wang X; Wang Y; Geng Y; Li F; Zheng C Isolation and purification of honokiol and magnolol from cortex Magnoliae Officinalis by high-speed counter-current chromatography. J. Chroma 2004, 1036, 171–175. - PubMed
-
- Esumi T; Makado G; Zhai H; Shimizu Y; Mitsumoto Y; Fukuyama Y Efficient synthesis and structure–activity relationship of honokiol, a neurotrophic biphenyl-type neolignane. Bioorg, Med. Chem. Lett 2004, 14, 2621–2625. - PubMed
- Harada K; Arioka C; Miyakita A; Kubo M; Kufuyama Y Efficient synthesis of neurotrophic honokiol using Suzuki–Miyaura reactions. Tetrahedron Lett. 2014, 55, 6001–6003.
-
- Srinivas J; Singh PP; Varma YK; Hyder I; Kumar HS Concise total synthesis of honokiol via Kumada cross coupling. Tetrahedron Lett. 2014, 55, 4295–4297.
-
- Jaracz S, Kozlowski MC, Lee YE, and Kim SM (April 27, 2017) Improved synthesis of Honokiol, WO/2017/070568.
Grants and funding
LinkOut - more resources
Full Text Sources
