Novel Alzheimer risk factor IQ motif containing protein K is abundantly expressed in the brain and is markedly increased in patients with Alzheimer's disease
- PMID: 35928571
- PMCID: PMC9344928
- DOI: 10.3389/fncel.2022.954071
Novel Alzheimer risk factor IQ motif containing protein K is abundantly expressed in the brain and is markedly increased in patients with Alzheimer's disease
Abstract
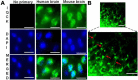
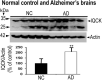
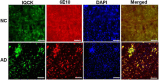
Alzheimer's disease (AD) is complex and highly heterogeneous. Less than 10% of AD cases are early-onset (EOAD) caused by autosomal dominantly inherited mutations in amyloid precursor protein (APP), presenilin 1 (PS1), or presenilin 2 (PS2), each of which can increase Aβ generation and, thus, amyloid plaques. The remaining 90% of cases of AD are late-onset (LOAD) or sporadic. Intense research efforts have led to identification of many genes that increase the risk of AD. An IQ motif containing protein K (IQCK) was recently identified by several investigators as an Alzheimer's disease risk gene. However, how IQCK increases AD risk is completely unknown. Since IQCK is a novel gene, there is limited information on its physiological characterization. To understand its role in AD, it is first important to determine its subcellular localization, whether and where it is expressed in the brain, and what type of brain cells express the IQCK protein. Therefore, in this study, we show by immunocytochemical (ICC) staining that IQCK is expressed in both the nucleus and the cytoplasm of SH-SY5Y neuroblastoma cells as well as HeLa cells but not in either HMC3 microglial or CHO cells. By immunohistochemistry (IHC), we also show that IQCK is expressed in both mouse and human neurons, including neuronal processes in vivo in the mouse brain. IHC data also show that the IQCK protein is widely expressed throughout the mouse brain, although regional differences were noted. IQCK expression was highest in the brainstem (BS), followed by the cerebellum (CB) and the cortex (CX), and it was lowest in the hippocampus (HP). This finding was consistent with data from an immunoblot analysis of brain tissue homogenates. Interestingly, we found IQCK expression in neurons, astrocytes, and oligodendrocytes using cell-specific antibodies, but IQCK was not detected in microglial cells, consistent with negative in vitro results in HMC3 cells. Most importantly, we found that actin-normalized IQCK protein levels were increased by 2 folds in AD brains relative to normal control (NC) brains. Furthermore, the IQCK protein was found in amyloid plaques, suggesting that IQCK may play a pathogenic role in either Aβ generation or amyloid plaque deposition in AD.
Keywords: Alzheimer’s disease; IQCK; astrocytes; brain regions; colocalization; microglia; neurons; oligodendrocytes.
Copyright © 2022 Wang, Devadoss, Nair, Chand and Lakshmana.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

