Long-Term Intranasal Nerve Growth Factor Treatment Favors Neuron Formation in de novo Brain Tissue
- PMID: 35928573
- PMCID: PMC9345199
- DOI: 10.3389/fncel.2022.871532
Long-Term Intranasal Nerve Growth Factor Treatment Favors Neuron Formation in de novo Brain Tissue
Abstract
Objective: To date, no safe and effective pharmacological treatment has been clinically validated for improving post-stroke neurogenesis. Growth factors are good candidates but low safety has limited their application in the clinic. An additional restraint is the delivery route. Intranasal delivery presents many advantages.
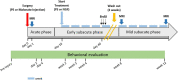
Materials and methods: A brain lesion was induced in twenty-four rats. Nerve growth factor (NGF) 5 μg/kg/day or vehicle was given intranasally from day 10 post-lesion for two periods of five weeks, separated by a two-week wash out period with no treatment. Lesion volume and atrophy were identified by magnetic resonance imaging (MRI). Anxiety and sensorimotor recovery were measured by behavior tests. Neurogenesis, angiogenesis and inflammation were evaluated by histology at 12 weeks.
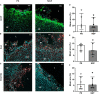
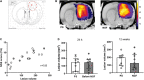
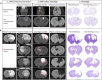
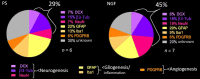
Results: Remarkable neurogenesis occurred and was visible at the second and third months after the insult. Tissue reconstruction was clearly detected by T2 weighted MRI at 8 and 12 weeks post-lesion and confirmed by histology. In the new tissue (8.1% of the lesion in the NGF group vs. 2.4%, in the control group at 12 weeks), NGF significantly increased the percentage of mature neurons (19% vs. 7%). Angiogenesis and inflammation were not different in the two groups. Sensorimotor recovery was neither improved nor hampered by NGF during the first period of treatment, but NGF treatment limited motor recovery in the second period.
Interpretation: The first five-week period of treatment was very well tolerated. This study is the first presenting the effects of a long treatment with NGF and has shown an important tissue regeneration rate at 8 and 12 weeks post-injury. NGF may have increased neuronal differentiation and survival and favored neurogenesis and neuron survival through subventricular zone (SVZ) neurogenesis or reprogramming of reactive astrocytes. For the first time, we evidenced a MRI biomarker of neurogenesis and tissue reconstruction with T2 and diffusion weighted imaging.
Keywords: MRI; acute brain lesion; intranasal; nerve growth factor; regeneration.
Copyright © 2022 Colitti, Desmoulin, Le Friec, Labriji, Robert, Michaux, Conchou, Cirillo and Loubinoux.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

