LIM Mineralization Protein-1 Enhances the Committed Differentiation of Dental Pulp Stem Cells through the ERK1/2 and p38 MAPK Pathways and BMP Signaling
- PMID: 35928717
- PMCID: PMC9346378
- DOI: 10.7150/ijms.70411
LIM Mineralization Protein-1 Enhances the Committed Differentiation of Dental Pulp Stem Cells through the ERK1/2 and p38 MAPK Pathways and BMP Signaling
Abstract
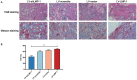
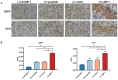
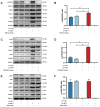
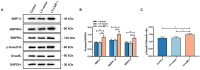
Tissue regeneration is the preferred treatment for dentin and bone tissue defects. Dental pulp stem cells (DPSCs) have been extensively studied for their use in tissue regeneration, including the regeneration of dentin and bone tissue. LIM mineralization protein-1 (LMP-1) is an intracellular non-secretory protein that plays a positive regulatory role in the mineralization process. In this study, an LMP-1-induced DPSCs model was used to explore the effect of LMP-1 on the proliferation and odonto/osteogenic differentiation of DPSCs, as well as the underlying mechanisms. As indicated by the cell counting kit-8 assay, the results showed that LMP-1 did not affect the proliferation of DPSCs. Overexpression of LMP-1 significantly promoted the committed differentiation of DPSCs and vice versa, as shown by alkaline phosphatase activity assay, alizarin red staining, western blot assay, quantitative real-time polymerase chain reaction assay, and in vivo mineralized tissue formation assay. Furthermore, inhibiting the activation of the extracellular signal-regulated kinase 1/2 (ERK1/2), p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK) pathways using specific pathway inhibitors showed that the ERK1/2 and p38 MAPK pathways attenuated the differentiation of DPSCs. Besides, the expression of BMP signaling pathway components were also determined, which suggested that LMP-1 could activate BMP-2/Smad1/5 signaling pathway. Our results not only indicated the underlying mechanism of LMP-1 treated DPSCs but also provided valuable insight into therapeutic strategies in regenerative medicine.
Keywords: Dental pulp stem cells; Differentiation; LIM mineralization protein-1; Mitogen-activated protein kinase pathway; Tissue regeneration.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
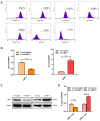
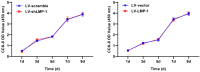
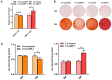
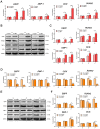
Similar articles
-
Calcium Hydroxide-induced Proliferation, Migration, Osteogenic Differentiation, and Mineralization via the Mitogen-activated Protein Kinase Pathway in Human Dental Pulp Stem Cells.J Endod. 2016 Sep;42(9):1355-61. doi: 10.1016/j.joen.2016.04.025. Epub 2016 Jul 7. J Endod. 2016. PMID: 27395474
-
Notoginsenoside R1 Promotes Osteogenic Differentiation of Dental Pulp Stem Cells via MAPK Pathway.Discov Med. 2025 Mar;37(194):486-495. doi: 10.24976/Discov.Med.202537194.40. Discov Med. 2025. PMID: 40116096
-
Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects.Stem Cell Res Ther. 2022 Feb 19;13(1):73. doi: 10.1186/s13287-022-02744-z. Stem Cell Res Ther. 2022. PMID: 35183254 Free PMC article.
-
Effects of different signaling pathways on odontogenic differentiation of dental pulp stem cells: a review.Front Physiol. 2023 Oct 19;14:1272764. doi: 10.3389/fphys.2023.1272764. eCollection 2023. Front Physiol. 2023. PMID: 37929208 Free PMC article. Review.
-
[Study and application of multidirectional differentiation potential of dental pulp stem cells].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2019 Feb 25;36(1):172-176. doi: 10.7507/1001-5515.201804045. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2019. PMID: 30887793 Free PMC article. Review. Chinese.
Cited by
-
Investigating the Role of Shikonin in Enhancing Osteogenesis and Angiogenesis for the Treatment of Osteoporosis.ACS Omega. 2025 Feb 27;10(9):9718-9727. doi: 10.1021/acsomega.4c11161. eCollection 2025 Mar 11. ACS Omega. 2025. PMID: 40092755 Free PMC article.
-
PDZ and LIM Domain-Encoding Genes: Their Role in Cancer Development.Cancers (Basel). 2023 Oct 19;15(20):5042. doi: 10.3390/cancers15205042. Cancers (Basel). 2023. PMID: 37894409 Free PMC article. Review.
-
Characterization of a Stemness-Optimized Purification Method for Human Dental-Pulp Stem Cells: An Approach to Standardization.Cells. 2022 Oct 12;11(20):3204. doi: 10.3390/cells11203204. Cells. 2022. PMID: 36291072 Free PMC article.
References
-
- Li M, Zhao Y, Hao H, Han W, Fu X. Mesenchymal stem cell-based therapy for nonhealing wounds: today and tomorrow. Wound Repair Regen. 2015;23:465–82. - PubMed
-
- Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–19. - PubMed
-
- Liao C, Zhou Y, Li M, Xia Y, Peng W. LINC00968 promotes osteogenic differentiation in vitro and bone formation in vivo via regulation of miR-3658/RUNX2. Differentiation. 2020;116:1–8. - PubMed
-
- Sabbagh J, Ghassibe-Sabbagh M, Fayyad-Kazan M, Al-Nemer F, Fahed JC, Berberi A. et al. Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. J Dent. 2020;101:103413. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

