CD106/VCAM-1 distinguishes a fibroblast subpopulation with high colony-forming capacity and distinct protein expression from the uterosacral ligament
- PMID: 35928741
- PMCID: PMC9347059
- DOI: 10.21037/atm-21-5136
CD106/VCAM-1 distinguishes a fibroblast subpopulation with high colony-forming capacity and distinct protein expression from the uterosacral ligament
Abstract
Background: Pelvic organ prolapse (POP) is a common degenerative disease in women which may diminish quality of life. Investigating the pathological changes of the uterosacral ligament, including the functional changes of fibroblasts, is critical to understanding the pathophysiology of POP. This study was designed to isolate CD106-positive (CD106+) fibroblasts from the human uterosacral ligament and assess the function and expression of this subpopulation.
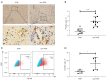
Methods: We separated CD106+ fibroblasts and CD106 negative (CD106-) fibroblasts by fluorescence-activated cell sorting (FACS) and cultured them for subsequent experiments. Flow cytometric analysis was used to test the sorting efficiency, CD106 expression, and typical mesenchymal stem cell (MSC) phenotype marker expression. A colony-forming unit (CFU) assay was applied to evaluate the colony-forming ability of the fibroblasts. Trilineage differentiation capacities were assessed after in vitro induction. The protein levels of vimentin, fibroblast specific protein-1 (FSP-1), collagen I (COL 1), matrix metallopeptidase-1 (MMP-1), and α-smooth muscle actin (α-SMA) were detected by western blot analysis. The expression of CD106 was verified by flow cytometric analysis and immunohistochemistry (IHC) in the POP and non-POP groups.
Results: The CD106+ fibroblasts were isolated with a purity of (93.50±3.91)%. The CD106+ fibroblasts exhibited higher colony-forming capacity than that of CD106- fibroblasts, but neither of them showed adipogenic or osteogenic differentiation similar to that of MSCs. The protein levels of MMP-1 and α-SMA were lower, and the level of COL 1 was higher in the CD106+ fibroblasts than in the CD106- fibroblasts. In addition, we observed a decreased expression of CD106 in the POP group compared with the non-POP group.
Conclusions: Our results suggest that CD106+ fibroblasts possess a high colony-forming capacity and distinct protein expression, and this subpopulation is reduced in POP.
Keywords: CD106; Fibroblasts; pelvic organ prolapse (POP); uterosacral ligament.
2022 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-5136/coif). The authors have no conflicts of interest to declare.
Figures






Similar articles
-
Treatment of Pelvic Organ Prolapse by the Downregulation of the Expression of Mitofusin 2 in Uterosacral Ligament Tissue via Mesenchymal Stem Cells.Genes (Basel). 2022 May 6;13(5):829. doi: 10.3390/genes13050829. Genes (Basel). 2022. PMID: 35627214 Free PMC article.
-
Relationship between the expressions of mitofusin-2 and procollagen in uterosacral ligament fibroblasts of postmenopausal patients with pelvic organ prolapse.Eur J Obstet Gynecol Reprod Biol. 2014 Mar;174:141-5. doi: 10.1016/j.ejogrb.2013.11.024. Epub 2013 Dec 7. Eur J Obstet Gynecol Reprod Biol. 2014. PMID: 24361166
-
Advanced glycation end products (AGEs) downregulate the miR-4429/PTEN axis to promote apoptosis of fibroblasts in pelvic organ prolapse.Ann Transl Med. 2022 Aug;10(15):821. doi: 10.21037/atm-22-628. Ann Transl Med. 2022. PMID: 36035012 Free PMC article.
-
Mechanical stress influences the morphology and function of human uterosacral ligament fibroblasts and activates the p38 MAPK pathway.Int Urogynecol J. 2022 Aug;33(8):2203-2212. doi: 10.1007/s00192-021-04850-7. Epub 2021 May 25. Int Urogynecol J. 2022. PMID: 34036402 Free PMC article.
-
Role of Fibroblasts and Myofibroblasts on the Pathogenesis and Treatment of Pelvic Organ Prolapse.Biomolecules. 2022 Jan 6;12(1):94. doi: 10.3390/biom12010094. Biomolecules. 2022. PMID: 35053242 Free PMC article. Review.
Cited by
-
Repurposing of the small-molecule adrenoreceptor-inhibitor carvedilol for treatment of the fibrotic lung.Front Pharmacol. 2025 May 22;16:1534989. doi: 10.3389/fphar.2025.1534989. eCollection 2025. Front Pharmacol. 2025. PMID: 40474967 Free PMC article.
-
Decellularization of Dense Regular Connective Tissue-Cellular and Molecular Modification with Applications in Regenerative Medicine.Cells. 2023 Sep 16;12(18):2293. doi: 10.3390/cells12182293. Cells. 2023. PMID: 37759515 Free PMC article. Review.
-
Surface Molecular Markers for the Isolation of Viable Fibroblast Subpopulations in the Female Reproductive Tract: A Comprehensive Review.Int J Mol Sci. 2024 Dec 30;26(1):233. doi: 10.3390/ijms26010233. Int J Mol Sci. 2024. PMID: 39796089 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
