HS-1793 inhibits cell proliferation in lung cancer by interfering with the interaction between p53 and MDM2
- PMID: 35928802
- PMCID: PMC9344265
- DOI: 10.3892/ol.2022.13410
HS-1793 inhibits cell proliferation in lung cancer by interfering with the interaction between p53 and MDM2
Abstract
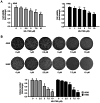
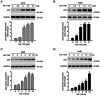
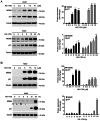
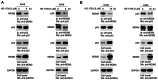
The transcription factor or tumor suppressor protein p53 regulates numerous cellular functions, including cell proliferation, invasion, migration, senescence and apoptosis, in various types of cancer. HS-1793 is an analog of resveratrol, which exhibits anti-cancer effects on various types of cancer, including breast, prostate, colon and renal cancer, and multiple myeloma. However, to the best of our knowledge, the role of HS-1793 in lung cancer remains to be examined. The present study aimed to investigate the anti-cancer effect of HS-1793 on lung cancer and to determine its association with p53. The results revealed that HS-1793 reduced cell proliferation in lung cancer and increased p53 stability, thereby elevating the expression levels of the target genes p21 and mouse double minute 2 homolog (MDM2). When the levels of MDM2, a negative regulator of p53, are increased under normal conditions, MDM2 binds and degrades p53; however, HS-1793 inhibited this binding, confirming that p53 protein stability was increased. In conclusion, the findings of the present study provide new evidence that HS-1793 may inhibit lung cancer proliferation by disrupting the p53-MDM2 interaction.
Keywords: HS-1793; MDM2; anti-cancer; lung cancer; p53.
Copyright: © Lim et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
