Using a Relative Quantitative Proteomic Method to Identify Differentially Abundant Proteins in Brucella melitensis Biovar 3 and Brucella melitensis M5-90
- PMID: 35928811
- PMCID: PMC9343586
- DOI: 10.3389/fimmu.2022.929040
Using a Relative Quantitative Proteomic Method to Identify Differentially Abundant Proteins in Brucella melitensis Biovar 3 and Brucella melitensis M5-90
Abstract
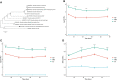
Brucellosis, caused by Brucella spp., is one of the most widespread bacterial zoonoses worldwide. Vaccination is still considered the best way to control brucellosis. An investigation into the differential proteome expression patterns of wild and vaccine strains may help researchers and clinicians differentiate between the strains to diagnose and better understand the mechanism(s) underlying differences in virulence. In the present study, a mass spectrometry-based, label-free relative quantitative proteomics approach was used to investigate the proteins expressed by the wild strain, B. melitensis biovar 3 and compare it with those expressed by B. melitensis M5-90. The higher level of virulence for B. melitensis biovar 3 compared to B. melitensis M5-90 was validated in vitro and in vivo. A total of 2133 proteins, encompassing 68% of the theoretical proteome, were identified and quantified by proteomic analysis, resulting in broad coverage of the B. melitensis proteome. A total of 147 proteins were identified as differentially expressed (DE) between these two strains. In addition, 9 proteins and 30 proteins were identified as unique to B. melitensis M5-90 and B. melitensis biovar 3, respectively. Pathway analysis revealed that the majority of the DE proteins were involved in iron uptake, quorum sensing, pyrimidine metabolism, glycine betaine biosynthetic and metabolic processes, thiamine-containing compound metabolism and ABC transporters. The expression of BtpA and VjbR proteins (two well-known virulence factors) in B. melitensis biovar 3 was 8-fold and 2-fold higher than in B. melitensis M5-90. In summary, our results identified many unique proteins that could be selected as candidate markers for differentiating vaccinated animals from animals with wild-type infections. BtpA and VjbR proteins might be responsible for the residual virulence of B. melitensis M5-90, while ABC transporters and thiamine metabolism associated proteins may be newly identified Brucella virulence factors. All of the identified DE proteins provide valuable information for the development of vaccines and the discovery of novel therapeutic targets.
Keywords: Brucella melitensis M5-90; Brucella melitensis biovar 3; Brucellosis; proteomics; vaccine.
Copyright © 2022 Zhang, Wang, Wang, Deng, Ji, Ma, Yang, Xu, Li, Yi, Wang, Wang, Sheng, Wang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential or actual conflict of interest.
Figures
Similar articles
-
Development and evaluation of in murine model, of an improved live-vaccine candidate against brucellosis from to Brucella melitensis vjbR deletion mutant.Microb Pathog. 2018 Nov;124:250-257. doi: 10.1016/j.micpath.2018.08.052. Epub 2018 Aug 25. Microb Pathog. 2018. PMID: 30149131
-
A Brucella melitensis M5-90 wboA deletion strain is attenuated and enhances vaccine efficacy.Mol Immunol. 2015 Aug;66(2):276-83. doi: 10.1016/j.molimm.2015.04.004. Epub 2015 Apr 17. Mol Immunol. 2015. PMID: 25899866
-
The Brucella melitensis M5-90ΔmanB live vaccine candidate is safer than M5-90 and confers protection against wild-type challenge in BALB/c mice.Microb Pathog. 2017 Nov;112:148-155. doi: 10.1016/j.micpath.2017.09.016. Epub 2017 Sep 12. Microb Pathog. 2017. PMID: 28916316
-
Incidence and control of brucellosis in the Near East region.Vet Microbiol. 2002 Dec 20;90(1-4):81-110. doi: 10.1016/s0378-1135(02)00248-1. Vet Microbiol. 2002. PMID: 12414137 Review.
-
Control of small ruminant brucellosis by use of Brucella melitensis Rev.1 vaccine: laboratory aspects and field observations.Vet Microbiol. 2002 Dec 20;90(1-4):497-519. doi: 10.1016/s0378-1135(02)00231-6. Vet Microbiol. 2002. PMID: 12414167 Review.
Cited by
-
Brucellae as resilient intracellular pathogens: epidemiology, host-pathogen interaction, recent genomics and proteomics approaches, and future perspectives.Front Vet Sci. 2023 Oct 9;10:1255239. doi: 10.3389/fvets.2023.1255239. eCollection 2023. Front Vet Sci. 2023. PMID: 37876633 Free PMC article. Review.
References
-
- Berhanu G, Pal M. Brucellosis: A Highly Infectious Zoonosis of Public Health and Economic Importance. J Environ Health Sci Eng (2020) 3:5–9.
-
- Liu FQ, Wang DL, Wang JQ, Li TF, Sen L. National Brucellosis Intervention Pilot County Survey on the Economic Losses. Chin J Control Endemic Dis (2008) 23:424–25. doi: 10.3969/j.issn.1001-1889.2008.06.008 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

