Rechallenge of denosumab in advanced giant cell tumor of the bone after atypical femur fracture: A case report and review of literature
- PMID: 35928864
- PMCID: PMC9343706
- DOI: 10.3389/fonc.2022.953149
Rechallenge of denosumab in advanced giant cell tumor of the bone after atypical femur fracture: A case report and review of literature
Abstract
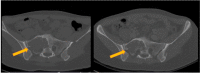
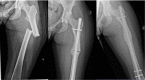
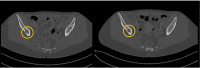
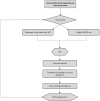
Giant cell tumor of the bone (GCTB) is a locally aggressive neoplasm where surgery is often curative. However, it can rarely give rise to distant metastases. Currently, the only available active therapeutic option for unresectable GCTB is denosumab, an anti-RANKL monoclonal antibody that dampens the aggressive osteolysis typically seen in this disease. For advanced/metastatic GCTB, denosumab should be continued lifelong, and although it is usually well tolerated, important questions may arise about the long-term safety of this drug. In fact, uncommon but severe toxicities can occur and eventually lead to denosumab discontinuation, such as atypical fracture of the femur (AFF). The optimal management of treatment-related AFF is a matter of debate, and to date, it is unknown whether reintroduction of denosumab at disease progression is a clinically feasible option, as no reports have been provided so far. Hereinafter, we present a case of a patient with metastatic GCTB who suffered from AFF after several years of denosumab; we describe the clinical features, orthopedic treatment, and oncological outcomes, finally providing the first evidence that denosumab rechallenge after AFF occurrence may be a safe and viable option at GCTB progression.
Keywords: atypical femur fracture; bone tumor; denosumab; giant cell tumor of bone; sarcoma.
Copyright © 2022 Nasca, Frezza, Morosi, Buonomenna, Parafioriti, Zappalà, Bini, Casali, Loppini and Stacchiotti.
Conflict of interest statement
AF, PC and SS perceived funds for institutional research and grants from Advenchen Laboratories, Amgen Dompé, AROG Pharmaceuticals, Bayer, Blueprint Medicines, Daiichi Sankyo, Deciphera, Eisai, Eli Lilly, Epizyme Inc, Glaxo, Karyopharm Pharmaceuticals, Novartis, Pfizer, PharmaMar. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Rechallenge of denosumab in jaw osteonecrosis of patients with unresectable giant cell tumour of bone: a case series analysis and literature review.ESMO Open. 2020 Jul;5(4):e000663. doi: 10.1136/esmoopen-2019-000663. ESMO Open. 2020. PMID: 32661185 Free PMC article. Review.
-
Denosumab treatment of inoperable or locally advanced giant cell tumor of bone - Multicenter analysis outside clinical trial.Eur J Surg Oncol. 2018 Sep;44(9):1384-1390. doi: 10.1016/j.ejso.2018.03.020. Epub 2018 Mar 31. Eur J Surg Oncol. 2018. PMID: 29650420 Clinical Trial.
-
Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study.Lancet Oncol. 2019 Dec;20(12):1719-1729. doi: 10.1016/S1470-2045(19)30663-1. Epub 2019 Nov 6. Lancet Oncol. 2019. PMID: 31704134 Clinical Trial.
-
Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): For how long?Eur J Cancer. 2017 May;76:118-124. doi: 10.1016/j.ejca.2017.01.028. Epub 2017 Mar 17. Eur J Cancer. 2017. PMID: 28324746
-
Denosumab in Giant Cell Tumor of Bone: Multidisciplinary Medical Management Based on Pathophysiological Mechanisms and Real-World Evidence.Cancers (Basel). 2022 May 4;14(9):2290. doi: 10.3390/cancers14092290. Cancers (Basel). 2022. PMID: 35565419 Free PMC article. Review.
Cited by
-
A Multicenter Study by the DENO Research Group on the Use of Denosumab in Giant-Cell Tumors of the Bone.J Clin Med. 2025 May 7;14(9):3242. doi: 10.3390/jcm14093242. J Clin Med. 2025. PMID: 40364272 Free PMC article.
-
Do Unresectable Giant Cell Tumors of Bone Treated With Denosumab Progress After Discontinuation of Treatment?Cancer Rep (Hoboken). 2025 Jan;8(1):e70117. doi: 10.1002/cnr2.70117. Cancer Rep (Hoboken). 2025. PMID: 39797695 Free PMC article.
-
Current Concepts in the Treatment of Giant Cell Tumor of Bone: An Update.Curr Oncol. 2024 Apr 8;31(4):2112-2132. doi: 10.3390/curroncol31040157. Curr Oncol. 2024. PMID: 38668060 Free PMC article. Review.

