A Phase II Study Evaluating the Interest to Combine UCPVax, a Telomerase CD4 TH1-Inducer Cancer Vaccine, and Atezolizumab for the Treatment of HPV Positive Cancers: VolATIL Study
- PMID: 35928870
- PMCID: PMC9343837
- DOI: 10.3389/fonc.2022.957580
A Phase II Study Evaluating the Interest to Combine UCPVax, a Telomerase CD4 TH1-Inducer Cancer Vaccine, and Atezolizumab for the Treatment of HPV Positive Cancers: VolATIL Study
Abstract
Background: There is a strong rational of using anti-programmed cell death protein-1 and its ligand (anti-PD-1/L1) antibodies in human papillomavirus (HPV)-induced cancers. However, anti-PD-1/L1 as monotherapy induces a limited number of objective responses. The development of novel combinations in order to improve the clinical efficacy of an anti-PD-1/L1 is therefore of interest. Combining anti-PD-1/L1 therapy with an antitumor vaccine seems promising in HPV-positive (+) cancers. UCPVax is a therapeutic cancer vaccine composed of two separate peptides derived from telomerase (hTERT, human telomerase reverse transcriptase). UCPVax is being evaluated in a multicenter phase I/II study in NSCLC (non-small cell lung cancer) and has demonstrated to be safe and immunogenic. The aim of the VolATIL study is to evaluate the combination of atezolizumab (an anti-PD-L1) and UCPVax vaccine in a multicenter phase II study in patients with HPV+ cancers.
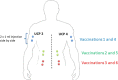
Methods: Patients with HPV+ cancer (anal canal, head and neck, and cervical or vulvar), at locally advanced or metastatic stage, and refractory to at least one line of systemic chemotherapy are eligible. The primary end point is the objective response rate (ORR) at 4 months. Patients will receive atezolizumab every 3 weeks at a fixed dose of 1,200 mg in combination with the UCPVax vaccine at 1 mg subcutaneously.
Discussion: Anti-cancer vaccines can restore cancer-immunity via the expansion and activation of tumor-specific T cells in patients lacking pre-existing anti-tumor responses. Moreover, preclinical data showed that specific TH1 CD4 T cells sustain the quality and homing of an antigen-specific CD8+ T-cell immunity. In previous clinical studies, the induction of anti-hTERT immunity was significantly correlated to survival in patients with advanced squamous anal cell carcinoma. Thus, there is a strong rational to combine an anti-cancer hTERT vaccine and an immune checkpoint inhibitor to activate and promote antitumor T-cell immunity. This pivotal proof of concept study will evaluate the efficacy and safety of the combination of a telomerase-based TH1 inducing vaccine (UCPVax) and an anti-PD-L1 (atezolizumab) immunotherapy in HPV+ cancers, as well as confirming their synergic mechanism, and settling the basis for a new combination for future clinical trials.
Clinical trial registration: https://www.clinicaltrials.gov/, identifier NCT03946358.
Keywords: HPV-related cancer; anal carcinoma; atezolizumab; cervix; head and neck; immunotherapy; vaccine.
Copyright © 2022 Rebucci-Peixoto, Vienot, Adotevi, Jacquin, Ghiringhelli, de la Fouchardière, You, Maurina, Kalbacher, Bazan, Meynard, Clairet, Fagnoni-Legat, Spehner, Bouard, Vernerey, Meurisse, Kim, Borg and Mansi.
Conflict of interest statement
CB: Research grant from Bayer and Roche, advisory board for Bayer, Sanofi, MSD. FB: Novartis, Seagen, Daiichi Sankyo, Pierre Fabre, Astra-Zeneca, Clovis. SK: reports a consulting or advisory role for Ipsen, Incyte, Boehringer Ingelheim, Sanofi and BeiGene and has received research funding from Pfizer, Roche, Novartis, Bristol-Myers Squibb, Boehringer Ingelheim and Sanofi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

