Linagliptin in Combination With Metformin Ameliorates Diabetic Osteoporosis Through Modulating BMP-2 and Sclerostin in the High-Fat Diet Fed C57BL/6 Mice
- PMID: 35928902
- PMCID: PMC9343600
- DOI: 10.3389/fendo.2022.944323
Linagliptin in Combination With Metformin Ameliorates Diabetic Osteoporosis Through Modulating BMP-2 and Sclerostin in the High-Fat Diet Fed C57BL/6 Mice
Abstract
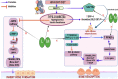
Background: Diabetic osteoporosis is a poorly managed serious skeletal complication, characterized by high fracture risk, increased bone resorption, reduced bone formation, and disrupted bone architecture. There is a need to investigate drugs that can improve bone health along with managing glycemic control. DPP-4 inhibitors and metformin have proven benefits in improving bone health. Here, we investigated the effects of linagliptin, a DPP inhibitor, and metformin alone and in combination to treat diabetic osteoporosis in high-fat-fed mice.
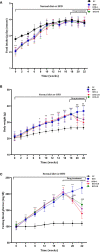
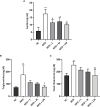
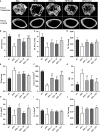
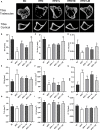
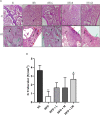
Methods: C57BL/6 mice were kept on the high-fat diet (HFD) for 22 weeks to induce diabetic osteoporosis. Linagliptin (10mg/Kg), metformin (150mg/Kg), and their combination were orally administered to the diabetic mice from the 18th-22nd week. Femur and tibial bone microarchitecture together with bone mineral density (BMD) were evaluated using µCT and histopathological changes were assessed. Further, bone turnover biomarkers namely bone morphogenetic protein-2 (BMP-2), sclerostin, tartrate-resistant acid phosphatase (TRAP), osteocalcin, alkaline phosphatase (ALP), calcium, and pro-inflammatory cytokines were assessed. Additionally, metabolic parameters including body weight, fasting blood glucose (FBG), glucose & insulin tolerance, lipids profile, and leptin were measured.
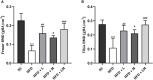
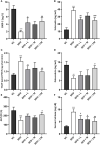
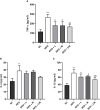
Results: HFD feeding resulted in impaired bone microarchitecture, reduced BMD, distorted bone histology, and altered bone turnover biomarkers as indicated by the significant reduction in bone ALP, BMP-2, osteocalcin, and an increase in sclerostin, TRAP, and serum calcium. Interestingly, treatment with linagliptin and its combination with metformin significantly reverted the impaired bone architecture, BMD, and positively modulated bone turnover biomarkers, while metformin alone did not exhibit any significant improvement. Further, HFD induced diabetes and metabolic abnormalities (including an increase in body weight, FBG, impaired glucose and insulin tolerance, leptin, triglycerides, cholesterol), and pro-inflammatory cytokines (TNF-alpha and IL-1β) were successfully reversed by treatment with linagliptin, metformin, and their combination.
Conclusion: Linagliptin and its combination with metformin successfully ameliorated diabetic osteoporosis in HFD-fed mice possibly through modulation of BMP-2 and sclerostin. The study provides the first evidence for the possible use of linagliptin and metformin combination for managing diabetic osteoporosis.
Keywords: DPP-4 inhibitors; bone architecture; diabetes; high fat diet (HFD); linagliptin; metformin; osteoporosis.
Copyright © 2022 Nirwan and Vohora.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Linagliptin-metformin combination: A novel approach to mitigate 4-vinyl cyclohexene di epoxide and dexamethasone-induced osteoporosis in mice.Bone. 2025 Sep;198:117526. doi: 10.1016/j.bone.2025.117526. Epub 2025 May 20. Bone. 2025. PMID: 40398630
-
Role of linagliptin in preventing the pathological progression of hepatic fibrosis in high fat diet and streptozotocin-induced diabetic obese rats.Eur J Pharmacol. 2020 Aug 15;881:173224. doi: 10.1016/j.ejphar.2020.173224. Epub 2020 May 23. Eur J Pharmacol. 2020. PMID: 32454118
-
Elevated T-helper 2 cytokine levels in high fat diet-fed C57BL/6 mice are attenuated by short-term 6-week treatment with a combination of low-dose aspirin and metformin.Cytokine. 2020 Apr;128:154999. doi: 10.1016/j.cyto.2020.154999. Epub 2020 Jan 31. Cytokine. 2020. PMID: 32014718
-
Sclerostin as a biomarker of physical exercise in osteoporosis: A narrative review.Front Endocrinol (Lausanne). 2022 Dec 5;13:954895. doi: 10.3389/fendo.2022.954895. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36545331 Free PMC article. Review.
-
The Potential Therapeutic Role of Metformin in Diabetic and Non-Diabetic Bone Impairment.Pharmaceuticals (Basel). 2022 Oct 17;15(10):1274. doi: 10.3390/ph15101274. Pharmaceuticals (Basel). 2022. PMID: 36297386 Free PMC article. Review.
Cited by
-
The role of lipid metabolism in osteoporosis: Clinical implication and cellular mechanism.Genes Dis. 2023 Sep 20;11(4):101122. doi: 10.1016/j.gendis.2023.101122. eCollection 2024 Jul. Genes Dis. 2023. PMID: 38523674 Free PMC article. Review.
-
The Expression Level of Inflammation-Related Genes in Patients With Bone Nonunion and the Effect of BMP-2 Infected Mesenchymal Stem Cells Combined With nHA/PA66 on the Inflammation Level of Femoral Bone Nonunion Rats.Physiol Res. 2024 Nov 19;73(5):819-829. doi: 10.33549/physiolres.935439. Physiol Res. 2024. PMID: 39560192 Free PMC article.
-
Anti-osteoporotic effect of sitagliptin in an osteoporosis model of ovariectomized rats: role of RUNX2 and RANKL/OPG ratio.Naunyn Schmiedebergs Arch Pharmacol. 2025 May 21. doi: 10.1007/s00210-025-04145-4. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40397119
-
Gut-Derived Peptide Hormone Analogues and Potential Treatment of Bone Disorders in Obesity and Diabetes Mellitus.Clin Med Insights Endocrinol Diabetes. 2024 Mar 13;17:11795514241238059. doi: 10.1177/11795514241238059. eCollection 2024. Clin Med Insights Endocrinol Diabetes. 2024. PMID: 38486712 Free PMC article. Review.
-
High-fat diet-induced osteoporosis in mice under hypoxic conditions.BMC Musculoskelet Disord. 2025 May 16;26(1):487. doi: 10.1186/s12891-025-08725-6. BMC Musculoskelet Disord. 2025. PMID: 40380162 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

