Increased TGFβ1 and SMAD3 Contribute to Age-Related Aortic Valve Calcification
- PMID: 35928937
- PMCID: PMC9343688
- DOI: 10.3389/fcvm.2022.770065
Increased TGFβ1 and SMAD3 Contribute to Age-Related Aortic Valve Calcification
Abstract
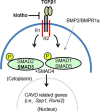
Aims: Calcific aortic valve disease (CAVD) is a progressive heart disease that is particularly prevalent in elderly patients. The current treatment of CAVD is surgical valve replacement, but this is not a permanent solution, and it is very challenging for elderly patients. Thus, a pharmacological intervention for CAVD may be beneficial. In this study, we intended to rescue aortic valve (AV) calcification through inhibition of TGFβ1 and SMAD3 signaling pathways.
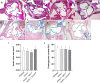
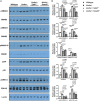
Methods and results: The klotho gene, which was discovered as an aging-suppressor gene, has been observed to play a crucial role in AV calcification. The klotho knockout (Kl -/-) mice have shorter life span (8-12 weeks) and develop severe AV calcification. Here, we showed that increased TGFβ1 and TGFβ-dependent SMAD3 signaling were associated with AV calcification in Kl -/- mice. Next, we generated Tgfb1- and Smad3-haploinsufficient Kl -/- mice to determine the contribution of TGFβ1 and SMAD3 to the AV calcification in Kl -/- mice. The histological and morphometric evaluation suggested a significant reduction of AV calcification in Kl -/-; Tgfb1 ± mice compared to Kl -/- mice. Smad3 heterozygous deletion was observed to be more potent in reducing AV calcification in Kl -/- mice compared to the Kl -/-; Tgfb1 ± mice. We observed significant inhibition of Tgfb1, Pai1, Bmp2, Alk2, Spp1, and Runx2 mRNA expression in Kl -/-; Tgfb1 ± and Kl -/-; Smad3 ± mice compared to Kl -/- mice. Western blot analysis confirmed that the inhibition of TGFβ canonical and non-canonical signaling pathways were associated with the rescue of AV calcification of both Kl -/-; Tgfb1 ± and Kl -/-; Smad3 ± mice.
Conclusion: Overall, inhibition of the TGFβ1-dependent SMAD3 signaling pathway significantly blocks the development of AV calcification in Kl -/- mice. This information is useful in understanding the signaling mechanisms involved in CAVD.
Keywords: CAVD (calcific aortic valve disease); Klotho; Smad3; Tgfb = transforming growth factor beta; aortic valve calcification.
Copyright © 2022 Chakrabarti, Bhattacharya, Gebere, Johnson, Ayub, Chatzistamou, Vyavahare and Azhar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
COX-2 Is Downregulated in Human Stenotic Aortic Valves and Its Inhibition Promotes Dystrophic Calcification.Int J Mol Sci. 2020 Nov 24;21(23):8917. doi: 10.3390/ijms21238917. Int J Mol Sci. 2020. PMID: 33255450 Free PMC article.
-
Bone Morphogenetic Protein Signaling Is Required for Aortic Valve Calcification.Arterioscler Thromb Vasc Biol. 2016 Jul;36(7):1398-405. doi: 10.1161/ATVBAHA.116.307526. Epub 2016 May 19. Arterioscler Thromb Vasc Biol. 2016. PMID: 27199449 Free PMC article.
-
NH4Cl Treatment Prevents Tissue Calcification in Klotho Deficiency.J Am Soc Nephrol. 2015 Oct;26(10):2423-33. doi: 10.1681/ASN.2014030230. Epub 2015 Feb 2. J Am Soc Nephrol. 2015. PMID: 25644113 Free PMC article.
-
In vitro 3D model and miRNA drug delivery to target calcific aortic valve disease.Clin Sci (Lond). 2017 Feb 1;131(3):181-195. doi: 10.1042/CS20160378. Clin Sci (Lond). 2017. PMID: 28057890 Free PMC article. Review.
-
Insights into calcific aortic valve stenosis: a comprehensive overview of the disease and advancing treatment strategies.Ann Med Surg (Lond). 2024 Apr 29;86(6):3577-3590. doi: 10.1097/MS9.0000000000002106. eCollection 2024 Jun. Ann Med Surg (Lond). 2024. PMID: 38846838 Free PMC article. Review.
Cited by
-
Mutations in fibulin-1 and collagen IV suppress the short healthspan of mig-17/ADAMTS mutants in Caenorhabditis elegans.PLoS One. 2024 Jul 9;19(7):e0305396. doi: 10.1371/journal.pone.0305396. eCollection 2024. PLoS One. 2024. PMID: 38980840 Free PMC article.
-
Molecular Interaction of Soluble Klotho with FGF23 in the Pathobiology of Aortic Valve Lesions Induced by Chronic Kidney Disease.Int J Biol Sci. 2024 Jun 17;20(9):3412-3425. doi: 10.7150/ijbs.92447. eCollection 2024. Int J Biol Sci. 2024. PMID: 38993571 Free PMC article.
-
TGF-β as A Master Regulator of Aging-Associated Tissue Fibrosis.Aging Dis. 2023 Oct 1;14(5):1633-1650. doi: 10.14336/AD.2023.0222. Aging Dis. 2023. PMID: 37196129 Free PMC article. Review.
References
-
- Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease-2011 update. Circulation. (2011) 124:1783–91. 10.1161/CIRCULATIONAHA.110.006767 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

