The Arabidopsis Rab protein RABC1 affects stomatal development by regulating lipid droplet dynamics
- PMID: 35929087
- PMCID: PMC9614440
- DOI: 10.1093/plcell/koac239
The Arabidopsis Rab protein RABC1 affects stomatal development by regulating lipid droplet dynamics
Abstract
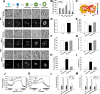
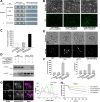
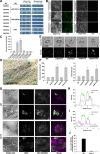
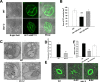
Lipid droplets (LDs) are evolutionarily conserved organelles that serve as hubs of cellular lipid and energy metabolism in virtually all organisms. Mobilization of LDs is important in light-induced stomatal opening. However, whether and how LDs are involved in stomatal development remains unknown. We show here that Arabidopsis thaliana LIPID DROPLETS AND STOMATA 1 (LDS1)/RABC1 (At1g43890) encodes a member of the Rab GTPase family that is involved in regulating LD dynamics and stomatal morphogenesis. The expression of RABC1 is coordinated with the different phases of stomatal development. RABC1 targets to the surface of LDs in response to oleic acid application in a RABC1GEF1-dependent manner. RABC1 physically interacts with SEIPIN2/3, two orthologues of mammalian seipin, which function in the formation of LDs. Disruption of RABC1, RABC1GEF1, or SEIPIN2/3 resulted in aberrantly large LDs, severe defects in guard cell vacuole morphology, and stomatal function. In conclusion, these findings reveal an aspect of LD function and uncover a role for lipid metabolism in stomatal development in plants.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R (2001) Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol 3: 531–537 - PubMed
-
- Bekbulat F, Schmitt D, Feldmann A, Huesmann H, Eimer S, Juretschke T, Beli P, Behl C, Kern A (2020) RAB18 loss interferes with lipid droplet catabolism and provokes autophagy network adaptations. J Mol Biol 432: 1216–1234 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

