TPC2 rescues lysosomal storage in mucolipidosis type IV, Niemann-Pick type C1, and Batten disease
- PMID: 35929194
- PMCID: PMC9449600
- DOI: 10.15252/emmm.202115377
TPC2 rescues lysosomal storage in mucolipidosis type IV, Niemann-Pick type C1, and Batten disease
Abstract
Lysosomes are cell organelles that degrade macromolecules to recycle their components. If lysosomal degradative function is impaired, e.g., due to mutations in lysosomal enzymes or membrane proteins, lysosomal storage diseases (LSDs) can develop. LSDs manifest often with neurodegenerative symptoms, typically starting in early childhood, and going along with a strongly reduced life expectancy and quality of life. We show here that small molecule activation of the Ca2+ -permeable endolysosomal two-pore channel 2 (TPC2) results in an amelioration of cellular phenotypes associated with LSDs such as cholesterol or lipofuscin accumulation, or the formation of abnormal vacuoles seen by electron microscopy. Rescue effects by TPC2 activation, which promotes lysosomal exocytosis and autophagy, were assessed in mucolipidosis type IV (MLIV), Niemann-Pick type C1, and Batten disease patient fibroblasts, and in neurons derived from newly generated isogenic human iPSC models for MLIV and Batten disease. For in vivo proof of concept, we tested TPC2 activation in the MLIV mouse model. In sum, our data suggest that TPC2 is a promising target for the treatment of different types of LSDs, both in vitro and in-vivo.
Keywords: Batten; MLIV; NPC1; TPC2; TRPML.
© 2022 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

- A
Colocalization of LacCer and LysoTracker (LyTr) in different CTR and LSD patient fibroblasts. Mander's coefficients were calculated using the Fiji JACoP plugin.
- B, C
Confocal images (B) and statistical analysis (C) showing colocalization of LacCer and LyTr in human CTR and MLIV fibroblasts, treated with TPC2‐A1‐P (30 μM, 16 h).
- D, E
Confocal images (D) and statistical analysis (E) showing colocalization of LacCer and LyTr in human CTR and NPC1 fibroblasts, treated with TPC2‐A1‐P (30 μM, 48 h).
- F, G
Confocal images and statistical analysis showing LacCer/LyTr colocalization in MLIV patient fibroblasts which were moc ‐electroporated and treated with DMSO or electroporated with a gain‐of‐function hTPC2(M484L/G734E):mCherry TOPO 3.1 vector and treated with either DMSO or TPC2‐A1‐P (30 μM, 16 h).
- H, I
Ca2+ chelation (BAPTA‐AM) dose dependently impairs LacCer trafficking in CTR fibroblasts.
- J, K
Confocal images (J) and statistical analysis (K) of NPC1 patient fibroblasts treated with 50 nM mock siRNA (siSCR) or siRNA targeting TPCN2 (siTPC2) for 72 h. Cells were then treated with DMSO or TPC2‐A1‐P (30 μM).

- A, B
Confocal images (A) and statistical analysis (B) of cholesterol accumulation in human CTR, MLIV, JNCL, and NPC1 fibroblasts. Cholesterol accumulation was evident for NPC1 and MLIV fibroblasts but not for JNCL fibroblasts. The images show filipin staining to visualize cholesterol accumulation and TO‐PRO3 as nuclear staining.
- C, D
TPC2‐A1‐P (30 μM, 48 h) rescued NPC1 and MLIV cholesterol accumulation.
- E–G
Confocal images (E‐F) and statistical analysis (G) of MLIV patient fibroblasts mock electroporated and treated with DMSO or electroporated with a gain‐of‐function hTPC2(M484L/G734E):mCherry TOPO 3.1 vector (white arrowheads) and treated with either DMSO or TPC2‐A1‐P (30 μM, 48 h).
- H–K
Confocal images (H‐I) and statistical analysis (J‐K) of human CTR and NPC1 patient fibroblasts treated with 50 nM mock siRNA (siSCR) or siRNA targeting TPCN2 (siTPC2) for 72 h. Cells were then treated with DMSO or TPC2‐A1‐P (30 μM).
- L, M
Statistics (L) and electron microscopy images (M) of human CTR, MLIV, JNCL, and NPC1 fibroblasts. The effect of the treatment with TPC2 agonist (30 μM, 48 h) was examined in NPC1 and MLIV cells.
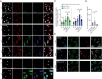
- A
Confocal images of CTR and JNCL fibroblasts. Images show LAMP1 staining and autofluorescence at 405 and 488 nm excitation wavelength, respectively, corresponding to the lipofuscin autofluorescence spectrum. Cells were treated with DMSO or TPC2‐A1‐P (30 μM) following cell cycle arrest (2 h mitomycin C treatment).
- B
Confocal images showing no autofluorescence signal at 594 nm excitation wavelength (used for LAMP1 staining).
- C
Mean autofluorescence intensity in LAMP1+ area.
- D, E
Confocal images of Gb3 accumulation stained with Shiga toxin (STX) in CTR and JNCL fibroblasts. Cells were treated with DMSO or TPC2‐A1‐P (30 μM) after cell cycle arrest.

- A–C
Cell viability assay for TPC2‐A1‐P and other compounds reported to activate TPC2 (Zhang et al, 2019) on human patient fibroblasts (A, B) and iPSC‐derived neurons (C). Cells were incubated for 24, 48, and 72 h with increasing compound concentrations, and cell viability was assessed with CellTiter‐Blue according to the manufacturer's protocol. Data are presented as mean ± SEM. n > 3 for each tested condition.

- A, B
Time course of filipin rescue (treatment with either DMSO or 30 μM TPC2‐A1‐P) in human MLIV and NPC1 fibroblasts (16–48 h).
- C, D
Ca2+ chelation (BAPTA‐AM) dose dependently causes cholesterol accumulation in CTR fibroblasts.
- E, F
Ca2+ chelation (BAPTA‐AM) blunts the effect of TPC2‐A1‐P (48 h treatment) when added for the last 3 h.
- G
RT‐qPCR showing TPCN2 knockdown efficiency in human CTR and NPC1 fibroblasts.

- A, B
Timeline of gene editing, quality control, and differentiation. The A18944 iPSC line (CTR) was used for gene editing. iPSCs were electroporated with a plasmid carrying spCas9, target gRNAs, and repair template. Target sites are shown in (B).
- C
Immunofluorescence images of pluripotency markers Tra1‐60, Oct4, SSEA4, and NANOG demonstrate pluripotency of CTR and gene‐edited iPSCs.
- D
Edited iPSCs were differentiated into cortical neurons expressing the neuronal markers TuJ and MAP2, and the cortical neuron transcription factor CTIP2.
- E
Using RT‐qPCR, we assessed the expression of lysosomal storage disease genes (TRPML1 for MLIV and CLN3 for JNCL) and drug targets (TRPMLs and TPCs).
- F, G
iPSC‐derived neurons were treated with apilimod to enlarge lysosomes, and TRPML1 responsiveness was assessed. ML‐SA1 (10 μM)‐elicited TRPML1 currents were observed in CTR lysosomes but not in MLIV neurons, indicative of abrogated TRPML1 function.
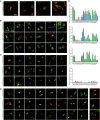
- A–D
Confocal images of CLN3 KO HeLa cells cotransfected with either GFP‐CLN3 CTR (WT) or GFP‐CLN3 missense mutant variants (as indicated) and endolysosomal markers: LAMP1‐RFP, Rab5‐RFP, or Rab11‐DsRed. Six mutants (B), CLN3S131R, CLN3C134R, CLN3A158P, CLN3G165E, CLN3L170P, and CLN3V330I, appeared strongly mislocalized to the cytosol. When present in patients (usually heterozygously, alongside the more prevalent CLN3Δ1.02kb variant), these variants reportedly result in a variety of clinical phenotypes, including classic JNCL, cone‐rod dystrophy, autophagic vacuolar myopathy, or retinitis pigmentosa (RP). A further six mutants (C), CLN3L101P, CLN3G189W, CLN3I285V, CLN3E295K, CLN3R334C, and CLN3Q352H showed no significant difference in colocalization with LAMP1, Rab5, or Rab11 compared to CTR CLN3. For these, clinical phenotypes have not been described, incompletely characterized, or described as protracted Batten disease or RP. The remaining nine mutations (D), CLN3G187A, CLN3G189R, CLN3G192E, CLN3V290L, CLN3L306H, CLN3V330F, CLN3R334H, CLN3R405W, and CLN3D416G showed significantly reduced lysosomal localization (LAMP1), while retaining endosomal localization. CLN3D416G also showed a significant decrease in Rab11 (recycling endosome) colocalization compared to CLN3 CTR. Rab5 (early endosome) colocalization was altered in four of these nine mutants, including CLN3D416G. Due to its consistent reduction in colocalization with all endolysosomal markers, CLN3D416G was chosen as a candidate for iPSC generation (with classic, more severe clinical JNCL phenotype).
- E
Quantification of experiments as shown in A‐D. Shown are the respective Mander's correlation coefficients (MCC) for automated colocalization analysis (JACoP/Fiji) of GFP‐CLN3 CTR and missense mutants with LAMP1‐RFP, Rab5‐RFP, Rab11‐DsRed, or MitoTracker‐DR (negative control).

- A
Four CLN3D416G iPSC clones were screened for locus copy numbers by qgPCR in comparison to the unedited parent line (black bar) to rule out undesired on‐target editing. The clone showing two CLN3 copies was selected (yellow bar).
- B
A heterozygous, silent SNP was found alongside the MLIVIVS3‐2A>G edit, confirming the presence of both edited alleles and ruling out large indels due to on‐target effects.
- C
Molecular karyotyping did not reveal any detectable aberrations in the selected cell lines at the targeted locus or chromosome 20, which is frequently altered in edited iPSCs.
- D
The most likely off‐target sites of the gRNAs used for each edit were predicted by CFD and MIT algorithms and sequenced, revealing no off‐target editing in the selected CLN3 and MLIV clones. The most likely off‐target sites for each clone are depicted.
- E
Tabular summary of sequenced off‐target sites.
- F, G
Effect of HBSS + TPC2‐A1‐P on LC3 in siSCR or siTPC2‐‐treated human CTR fibroblasts.
- H–K
Effect of TPC2‐A1‐P on P62 accumulation with and without bafilomycin A1 treatment in human NPC1 and MLIV fibroblasts.
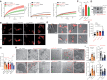
- A, B
Lysosomal proteolysis was measured following pre‐treatment with DMSO/TPC2‐A1‐P, using the magic red (MR) cathepsin B substrate, and performing FRAP measurements to assess the proteolysis rate. MCOLN1IVS3‐2A>G (MLIV) neurons showed increased proteolysis, while CLN3D416G and CLN3ΔEx4–7 neurons (JNCL) exhibited either significantly lower or slightly reduced proteolysis rates, respectively.
- C, D
Western blot analysis of cathepsin B (CtsB) in CTR and MCOLN1IVS3‐2A>G neurons treated with TPC2‐A1‐P (30 μM) or DMSO.
- E–G
Cortical neurons were treated with compounds and acidic compartments stained with LysoTracker (LyTr). The endolysosomal expansion was observed in MCOLN1IVS3‐2A>G, CLN3D416G, and CLN3ΔEx4–7 neurons, which was ameliorated by TPC2‐A1‐P (30 μM) treatment.
- H, I
Electron microscopy analysis of neuronal rosettes (neuronal progenitor cells, NPC) treated with DMSO or TPC2‐A1‐P. TPC2‐A1‐P treatment significantly decreased the number of inclusion bodies (black arrowheads) in MCOLN1IVS3‐2A>G. CLN3D416G and CLN3ΔEx4–7 lacked an appropriate assay window and showed no significant accumulation of inclusion bodies. However, CLN3ΔEx4–7 NPC showed significantly more mitochondria with aberrant cristae numbers (white arrowheads), a phenotype which was rescued by TPC2‐A1‐P (30 μM) treatment.

- A
Confocal images of plasma membrane (PM) LAMP1 immunofluorescence in CTR fibroblasts. LAMP1 on the PM is expressed as fold change relative to DMSO‐treated cells.
- B
Statistical analysis of lysosomal exocytosis data as shown in (A).
- C
Lysosomal exocytosis in CTR, MLIV, NPC1, and JNCL human fibroblasts. PM‐localized LAMP1 was measured by flow cytometry, expressed as percent of CTR DMSO‐treated cells. Ionomycin in (A–C) (4 μM; 10 min treatment) was used as a positive control. TPC2‐A1‐P and ML‐SA1 (30 μM, each; 90 min treatment in A–C).
- D
Cartoon showing lysosomal exocytosis. Statistics (B, C): Shown are mean values ± SEM. n > 3 for each tested condition (in (B), each dot represents an imaged frame containing several cells, and in (C) each dot is the mean FITC intensity value expressed as a percentage obtained from at least 1 × 104 events); two‐way ANOVA, post hoc Dunnett's (B), or Tukey's (C) multiple comparisons test; *p‐value < 0.05; ***p‐value < 0.001; ****p‐value < 0.0001.
- E
Cartoon showing the roles of LC3 and P62 in the autophagic pathway.
- F, G
Immunoblot analysis of endogenous LC3 (LC3I‐II) following TPC2‐A1‐P or ML‐SA1 (30 μM, each) treatment, alone or with BafA1, under fed (complete media), or starvation (HBSS) conditions in CTR, MLIV, and NPC1 patient fibroblasts. Graphs show densitometry of LC3II bands normalized to actin.
- H
Immunoblot analysis of endogenous LC3 (LC3I‐II) following TPC2‐A1‐P (30 μM) or DMSO treatment, under fed (complete neurobasal/B27), or starvation (DMEM/F12 free) conditions in CTR and MLIV iPSC‐derived cortical neurons. Graphs show densitometry of LC3II bands normalized to actin.
- I, J
Immunoblot and statistical analysis of endogenous SQSTM1 (P62) upon TPC2‐A1‐P or ML‐SA1 (30 μM, each) treatment, under fed (complete media), or starvation (HBSS) conditions in CTR, MLIV, and NPC1 patient fibroblasts.

- A–B
Both neurons and glia were found to express Tpc2 in the corpus callosum, the hippocampus, and the cerebellum (so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum; mo, molecular layer; gr, granular layer). Subpopulations of astrocytes (Gfap) and microglia (Iba1) express Tpc2 (E).
- C
One week (1w)‐ or 8‐week (8w)‐old mouse brains were dissected, and brain Tpc2 transcript was mapped.
- D
A cDNA array was used to map TPC2 transcripts in the human brain.
- E
Quantification of astrocytes(Gfap), microglia (Iba1) and neurons in different brain areas and percentage of cells coexpressing Tpc2 and the respective marker (Dbl = double labeled).
- F
Tpc2 expression in the Tpc2 reporter mouse, mouse brain, and human brain is summarized as cartoons, finding highest expression in cerebellum and hippocampus (top panels). Affected brain regions in the lysosomal storage diseases MLIV, JNCL, and NPC1 based on patient and mouse data are color-coded.
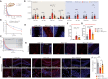
- A
TPC2‐A1‐P was injected intravenously, and mice were sacrificed at the indicated time. TPC2‐A1‐P was measured in plasma and brain by LC–MS/MS. TPC2‐A1‐P was rapidly eliminated, being undetectable by 240 min.
- B
Elimination rate constants were determined using a semi‐log plot. A two‐phase decay model could fit the obtained data points in plasma, while a one‐phase decay model fits the data in the brain.
- C
Brain [TPC2‐A1‐P] was simulated for various injected doses. 20 mg/kg TPC2‐A1‐P was chosen to avoid off‐target activity while providing a therapeutic dose for > 20 min.
- D, E
From 2 months of age, MLIV mice were injected daily with TPC2‐A1‐P i.p. and sacrificed after 13 weeks; av, arbor vitae; gr, granular cell layer; slm, stratum lacunosum moleculare; and sp, stratum pyramidale. Mild microgliosis (Iba1) was observed only in the cerebellar av, while astrogliosis (Gfap) was observed in the cerebellar av and gr layers in MLIV mice. TPC2‐A1‐P ameliorated MLIV‐associated cerebellar astrogliosis.
- F, G
Plots showing mean numbers of P62 aggregates per section (cerebellum (F) and hippocampus (G)).
- H, I
Confocal images of endogenous P62/SQSTM1 inclusion in CTR and MLIV mouse cerebellar coronal (H) and hippocampal (I) sections.
- J
Results of the rotarod experiments using MLIV mice treated with vehicle or TPC2‐A1‐P, respectively, compared to vehicle‐treated WT littermates (CTR).
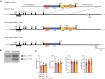
- A
Targeting strategy used to express Cre recombinase under the control of the Tpcn2 promoter. The targeting vector contains an IRES‐Cre‐FRT‐PGK‐NEO‐FRT cassette in which a phosphoglycerate kinase promoter drives neomycin resistance (Pgk‐neo). This cassette is incorporated by homologous recombination in embryonic stem cells subsequent to the stop codon in exon 25.
- B
Southern blot of embryonic stem cell DNA cut with DrdI, demonstrating correct targeting of the Tpcn2‐IRES‐Cre knock‐in allele.
- C
Results of the open‐field test using MLIV mice treated with vehicle or TPC2‐A1‐P, respectively, compared to vehicle‐treated WT littermates (CTR). No differences between CTR and MLIV mice were observed.
References
-
- Bargal R, Avidan N, Olender T, Ben AE, Zeigler M, Raas‐Rothschild A, Frumkin A, Ben‐Yoseph O, Friedlender Y, Lancet D et al (2001) Mucolipidosis type IV: novel MCOLN1 mutations in Jewish and Non‐Jewish patients and the frequency of the disease in the Ashkenazi Jewish population. Hum Mutat 17: 397–402 - PubMed
-
- Bretou M, Sáez PJ, Sanséau D, Maurin M, Lankar D, Chabaud M, Spampanato C, Malbec O, Barbier L, Muallem S et al (2017) Lysosome signaling controls the migration of dendritic cells. Sci Immunol 2: eaak9573 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

