Leadless or Conventional Transvenous Ventricular Permanent Pacemakers: A Nationwide Matched Control Study
- PMID: 35929449
- PMCID: PMC9496294
- DOI: 10.1161/JAHA.122.025339
Leadless or Conventional Transvenous Ventricular Permanent Pacemakers: A Nationwide Matched Control Study
Abstract
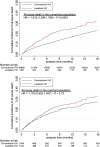
Background Leadless ventricular permanent pacemakers (leadless VVI or LPM) were designed to reduce lead-related complications of conventional VVI pacemakers (CPM). The aim of our study was to assess and compare real-life clinical outcomes within the first 30 days and during a midterm follow-up with the 2 techniques. Methods and Results This French longitudinal cohort study was based on the national hospitalization database. All adults (age ≥18 years) hospitalized in French hospitals from January 1, 2017 to September 1, 2020, who underwent a first LPM or CPM were included. The study included 40 828 patients with CPM and 1487 with LPM. After propensity score matching 1344 patients with CPM were matched 1:1 with patients treated with LPM. Patients with LPM had a lower rate of all-cause and cardiovascular death within the 30 days after implantation. During subsequent follow-up (mean: 8.6±10.5 months), risk of all-cause death in the unmatched population was significantly higher in the LPM group than in the CPM group, whereas risk of cardiovascular death and of endocarditis was not significantly different. After matching on all baseline characteristics including comorbidities (mean follow-up 6.2±8.7 months), all-cause death, cardiovascular death, and infective endocarditis were not statistically different in the 2 groups. Conclusions Patients treated with leadless VVI pacemakers had better clinical outcomes in the first month compared with the patients treated with conventional VVI pacing. During a midterm follow-up, risk of all-cause death, cardiovascular death, and endocarditis in patients treated with leadless VVI pacemaker was not statistically different after propensity score matching.
Keywords: leadless; pacemakers; transvenous.
Figures



Similar articles
-
Comparison of in-hospital outcomes and complications of leadless pacemaker and traditional transvenous pacemaker implantation.Europace. 2023 Aug 2;25(9):euad269. doi: 10.1093/europace/euad269. Europace. 2023. PMID: 37712644 Free PMC article.
-
Improved outcomes with leadless vs. single-chamber transvenous pacemaker in haemodialysis patients.Europace. 2024 Nov 1;26(11):euae257. doi: 10.1093/europace/euae257. Europace. 2024. PMID: 39351810 Free PMC article.
-
Contemporaneous Comparison of Outcomes Among Patients Implanted With a Leadless vs Transvenous Single-Chamber Ventricular Pacemaker.JAMA Cardiol. 2021 Oct 1;6(10):1187-1195. doi: 10.1001/jamacardio.2021.2621. JAMA Cardiol. 2021. PMID: 34319383 Free PMC article.
-
Permanent Leadless Cardiac Pacemaker Therapy: A Comprehensive Review.Circulation. 2017 Apr 11;135(15):1458-1470. doi: 10.1161/CIRCULATIONAHA.116.025037. Circulation. 2017. PMID: 28396380 Review.
-
Leadless Cardiac Pacemakers: Pacing Paradigm Change.Curr Cardiol Rep. 2015 Aug;17(8):68. doi: 10.1007/s11886-015-0619-3. Curr Cardiol Rep. 2015. PMID: 26233700 Review.
Cited by
-
Implantable cardiac monitor and leadless pacemaker in the management of syncope due to intermittent high-degree atrioventricular block: a case report.J Cardiothorac Surg. 2024 Jul 13;19(1):443. doi: 10.1186/s13019-024-02962-x. J Cardiothorac Surg. 2024. PMID: 39003494 Free PMC article.
-
Systematic Review and Meta-Analysis of Acute Mortality and Complication Rates Following Leadless Pacemaker Placement Using National-Level Data.Medicina (Kaunas). 2025 May 25;61(6):974. doi: 10.3390/medicina61060974. Medicina (Kaunas). 2025. PMID: 40572663 Free PMC article.
-
The Effectiveness and Safety of Leadless Pacemakers: An Updated Meta-Analysis.Curr Cardiol Rep. 2024 Aug;26(8):789-799. doi: 10.1007/s11886-024-02079-6. Epub 2024 Jun 13. Curr Cardiol Rep. 2024. PMID: 38869811
-
Implantable Cardiac Devices in Patients with Brady- and Tachy-Arrhythmias: An Update of the Literature.Rev Cardiovasc Med. 2024 May 11;25(5):162. doi: 10.31083/j.rcm2505162. eCollection 2024 May. Rev Cardiovasc Med. 2024. PMID: 39076493 Free PMC article. Review.
-
Comparison of Postoperative Outcomes between Leadless and Conventional Transvenous Pacemakers Implantation: An Up-to-Date Meta-analysis.Rev Cardiovasc Med. 2024 Oct 9;25(10):359. doi: 10.31083/j.rcm2510359. eCollection 2024 Oct. Rev Cardiovasc Med. 2024. PMID: 39484120 Free PMC article.
References
-
- Clémenty N, Carion PL, Léotoing L, Lamarsalle L, Wilquin‐Bequet F, Brown B, Verhees KJP, Fernandes J, Deharo J‐C. Infections and associated costs following cardiovascular implantable electronic device implantations: a nationwide cohort study. Europace. 2018;20:1974–1980. doi: 10.1093/europace/eux387 - DOI - PubMed
-
- Roberts PR, Clementy N, Al Samadi F, Garweg C, Martinez‐Sande JL, Iacopino S, Johansen JB, Vinolas Prat X, Kowal RC, Klug D, et al. A leadless pacemaker in the real‐world setting: the Micra transcatheter pacing system post‐approval registry. Heart Rhythm. 2017;14:1375–1379. doi: 10.1016/j.hrthm.2017.05.017 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

