Repeated Measures of Modified Rankin Scale Scores to Assess Functional Recovery From Stroke: AFFINITY Study Findings
- PMID: 35929466
- PMCID: PMC9496315
- DOI: 10.1161/JAHA.121.025425
Repeated Measures of Modified Rankin Scale Scores to Assess Functional Recovery From Stroke: AFFINITY Study Findings
Abstract
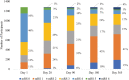
Background Function after acute stroke using the modified Rankin Scale (mRS) is usually assessed at a point in time. The analytical implications of serial mRS measurements to evaluate functional recovery over time is not completely understood. We compare repeated-measures and single-measure analyses of the mRS from a randomized clinical trial. Methods and Results Serial mRS data from AFFINITY (Assessment of Fluoxetine in Stroke Recovery), a double-blind placebo randomized clinical trial of fluoxetine following stroke (n=1280) were analyzed to identify demographic and clinical associations with functional recovery (reduction in mRS) over 12 months. Associations were identified using single-measure (day 365) and repeated-measures (days 28, 90, 180, and 365) partial proportional odds logistic regression. Ninety-five percent of participants experienced a reduction in mRS after 12 months. Functional recovery was associated with age at stroke <70 years; no prestroke history of diabetes, coronary heart disease, or ischemic stroke; prestroke history of depression, a relationship partner, living with others, independence, or paid employment; no fluoxetine intervention; ischemic stroke (compared with hemorrhagic); stroke treatment in Vietnam (compared with Australia or New Zealand); longer time since current stroke; and lower baseline National Institutes of Health Stroke Scale & Patient Health Questionnaire-9 scores. Direction of associations was largely concordant between single-measure and repeated-measures models. Association strength and variance was generally smaller in the repeated-measures model compared with the single-measure model. Conclusions Repeated-measures may improve trial precision in identifying trial associations and effects. Further repeated-measures stroke analyses are required to prove methodological value. Registration URL: http://www.anzctr.org.au; Unique identifier: ACTRN12611000774921.
Keywords: cerebrovascular disease; functional outcomes; modified Rankin Scale; partial proportional odds; repeated measures; stroke.
Figures
Similar articles
-
Twelve-Month Outcomes of the AFFINITY Trial of Fluoxetine for Functional Recovery After Acute Stroke: AFFINITY Trial Steering Committee on Behalf of the AFFINITY Trial Collaboration.Stroke. 2021 Aug;52(8):2502-2509. doi: 10.1161/STROKEAHA.120.033070. Epub 2021 May 21. Stroke. 2021. PMID: 34015940 Clinical Trial.
-
Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial.Lancet Neurol. 2020 Aug;19(8):651-660. doi: 10.1016/S1474-4422(20)30207-6. Lancet Neurol. 2020. PMID: 32702334 Clinical Trial.
-
Depression Outcomes Among Patients Treated With Fluoxetine for Stroke Recovery: The AFFINITY Randomized Clinical Trial.JAMA Neurol. 2021 Sep 1;78(9):1072-1079. doi: 10.1001/jamaneurol.2021.2418. JAMA Neurol. 2021. PMID: 34338714 Free PMC article. Clinical Trial.
-
The FOCUS, AFFINITY and EFFECTS trials studying the effect(s) of fluoxetine in patients with a recent stroke: a study protocol for three multicentre randomised controlled trials.Trials. 2015 Aug 20;16:369. doi: 10.1186/s13063-015-0864-1. Trials. 2015. PMID: 26289352 Free PMC article. Clinical Trial.
-
[Therapies for the Improvement of Stroke Recovery - Assessment of Clinical Trial Results].Fortschr Neurol Psychiatr. 2023 Dec;91(12):516-522. doi: 10.1055/a-2181-1026. Epub 2023 Dec 11. Fortschr Neurol Psychiatr. 2023. PMID: 38081165 Review. German.
Cited by
-
Disability and Recurrent Stroke Among Participants in Stroke Prevention Trials.JAMA Netw Open. 2024 Jul 1;7(7):e2423677. doi: 10.1001/jamanetworkopen.2024.23677. JAMA Netw Open. 2024. PMID: 39028666 Free PMC article.
-
Personalized Post-Stroke Rehabilitation in a Rural Community: A Pilot Quasi-Experimental Study on Activities of Daily Living and Disability Outcomes Using Participatory Action Research.Healthcare (Basel). 2025 May 28;13(11):1275. doi: 10.3390/healthcare13111275. Healthcare (Basel). 2025. PMID: 40508888 Free PMC article.
-
Revisiting flow augmentation bypass for cerebrovascular atherosclerotic vaso-occlusive disease: Single-surgeon series and review of the literature.PLoS One. 2023 May 19;18(5):e0285982. doi: 10.1371/journal.pone.0285982. eCollection 2023. PLoS One. 2023. PMID: 37205640 Free PMC article. Review.
-
The real-life reliability of the modified Rankin scale used in a stroke unit and a rehabilitation ward.Postep Psychiatr Neurol. 2025 Mar;34(1):19-25. doi: 10.5114/ppn.2025.149879. Epub 2025 Apr 30. Postep Psychiatr Neurol. 2025. PMID: 40376289 Free PMC article.
-
Association of Anaemia With Higher Mortality and Disability After Subarachnoid Haemorrhage: A Single-Centre Cohort Study.Cureus. 2025 Apr 26;17(4):e83031. doi: 10.7759/cureus.83031. eCollection 2025 Apr. Cureus. 2025. PMID: 40421344 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical