Effect of Canagliflozin on Total Cardiovascular Burden in Patients With Diabetes and Chronic Kidney Disease: A Post Hoc Analysis From the CREDENCE Trial
- PMID: 35929472
- PMCID: PMC9496296
- DOI: 10.1161/JAHA.121.025045
Effect of Canagliflozin on Total Cardiovascular Burden in Patients With Diabetes and Chronic Kidney Disease: A Post Hoc Analysis From the CREDENCE Trial
Abstract
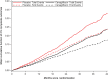
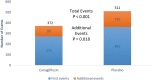
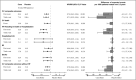
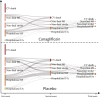
Background The sodium-glucose cotransporter 2 inhibitor canagliflozin reduced the risk of first cardiovascular composite events in the CREDENCE (Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation) trial. In this post hoc analysis, we evaluated the effect of canagliflozin on total (first and recurrent) cardiovascular events. Methods and Results The CREDENCE trial compared canagliflozin or matching placebo in 4401 patients with type 2 diabetes, albuminuria, and estimated glomerular filtration rate of 30 to <90 mL/min per 1.73 m2, over a median of 2.6 years. The primary outcome was analyzed as a composite of any cardiovascular event including myocardial infarction, stroke, hospitalization for heart failure, hospitalization for unstable angina, and cardiovascular death. Negative binomial regression models were used to assess the effect of canagliflozin on the net burden of cardiovascular events. During the trial, 634 patients had 883 cardiovascular events, of whom 472 (74%) had just 1 cardiovascular event and 162 (26%) had multiple cardiovascular events. Canagliflozin reduced first cardiovascular events by 26% (hazard ratio, 0.74 [95% CI, 0.63-0.86]; P<0.001) and total cardiovascular events by 29% (incidence rate ratio, 0.71 [95% CI, 0.59-0.86]; P<0.001). The absolute risk difference per 1000 patients treated over 2.5 years was -44 (95% CI, -67 to -21) first cardiovascular events and -73 (95% CI, -114 to -33) total events. Conclusions Canagliflozin reduced cardiovascular events, with a larger absolute benefit for total cardiovascular than first cardiovascular events. These findings provide further support for the benefit of continuing canagliflozin therapy after an initial event to prevent recurrent cardiovascular events. Registration Information URL: https://www.clinicaltrials.gov; Unique Identifier: NCT02065791.
Keywords: canagliflozin; chronic kidney disease; diabetes; recurrent cardiovascular event.
Figures
References
-
- Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, Rodgers A, Mahaffey KW, Cannon CP, Perkovic V, et al. Sodium‐glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta‐analysis. J Am Heart Assoc. 2020;9:e014908. doi: 10.1161/JAHA.119.014908 - DOI - PMC - PubMed
-
- McMurray JJV, Wheeler DC, Stefánsson BV, Jongs N, Postmus D, Correa‐Rotter R, Chertow GM, Greene T, Held C, Hou F‐F, et al. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation. 2021;143:438–448. doi: 10.1161/CIRCULATIONAHA.120.051675 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

