Interconnection between cardiovascular, renal and metabolic disorders: A narrative review with a focus on Japan
- PMID: 35929483
- PMCID: PMC9804928
- DOI: 10.1111/dom.14829
Interconnection between cardiovascular, renal and metabolic disorders: A narrative review with a focus on Japan
Abstract
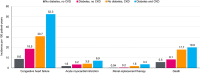
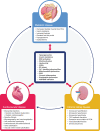
Insights from epidemiological, clinical and basic research are illuminating the interplay between metabolic disorders, cardiovascular disease (CVD) and kidney dysfunction, termed cardio-renal-metabolic (CRM) disease. Broadly defined, CRM disease involves multidirectional interactions between metabolic diseases such as type 2 diabetes (T2D), various types of CVD and chronic kidney disease (CKD). T2D confers increased risk for heart failure, which-although well known-has only recently come into focus for treatment, and may differ by ethnicity, whereas atherosclerotic heart disease is a well-established complication of T2D. Many people with T2D also have CKD, with a higher risk in Asians than their Western counterparts. Furthermore, CVD increases the risk of CKD and vice versa, with heart failure, notably, present in approximately half of CKD patients. Molecular mechanisms involved in CRM disease include hyperglycaemia, insulin resistance, hyperactivity of the renin-angiotensin-aldosterone system, production of advanced glycation end-products, oxidative stress, lipotoxicity, endoplasmic reticulum stress, calcium-handling abnormalities, mitochondrial malfunction and deficient energy production, and chronic inflammation. Pathophysiological manifestations of these processes include diabetic cardiomyopathy, vascular endothelial dysfunction, cardiac and renal fibrosis, glomerular hyperfiltration, renal hypoperfusion and venous congestion, reduced exercise tolerance leading to metabolic dysfunction, and calcification of atherosclerotic plaque. Importantly, recognition of the interaction between CRM diseases would enable a more holistic approach to CRM care, rather than isolated treatment of individual conditions, which may improve patient outcomes. Finally, aspects of CRM diseases may differ between Western and East Asian countries such as Japan, a super-ageing country, with potential differences in epidemiology, complications and prognosis that represent an important avenue for future research.
Keywords: cardiovascular disease; diabetic nephropathy; heart failure; type 2 diabetes.
© 2022 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
T.K. reports consulting/lecture fees from Abbott, Asahi Mutual Life Insurance, Astellas Pharma Inc., AstraZeneca K.K., Bayer, Boehringer Ingelheim, Cosmic, Daiichi Sankyo Company, Limited, Eli Lilly and Company, Fujifilm, FUJIREBIO, Johnson & Johnson Co., Ltd, Kissei Pharmaceutical Co., Ltd, Kowa Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Medical Review, Medscape Education, Medtronic Sofamor Danek, Mitsubishi Tanabe Pharma Corporation, MSD, Musashino Foods, Nipro, Novartis International AG, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Sanofi S.A., SANWA KAGAKU KENKYUSHO CO., LTD, Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd, Takeda Pharmaceutical Company Limited and Terumo; grants from Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Eli Lilly and Company, Kissei Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Sanofi S.A., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Company Limited; contracted research from AstraZeneca K.K. and Takeda Pharmaceutical Company Limited; joint research from Daiichi Sankyo Company, Limited; endowed chair from Asahi Mutual Life Insurance, Boehringer Ingelheim, Kowa Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Company Limited. H.M. has received lecture fees from MSD K.K., Nippon Boehringer Ingelheim Co. Ltd, Astellas Pharma Inc., Mitsubishi‐Tanabe Pharma Corporation, Sanofi K.K., Takeda Pharmaceutical Co. Ltd, Daiichi Sankyo Co. Ltd, AstraZeneca K.K., Novo Nordisk Pharma Ltd, Sumitomo Dainippon Pharma Co. Ltd and Eli Lilly; research support from Astellas Pharma Inc., AstraZeneca K.K., Nippon Boehringer Ingelheim Co. Ltd, Sunstar Inc., Mitsubishi Tanabe Pharma Corporation, Kyowa Kirin Co. Ltd, Nissan Chemical Corporation and MIKI Corporation; and research grants from Takeda Pharmaceutical Co. Ltd, Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd, Kyowa Kirin Co. Ltd, Taisho‐Toyama Pharm Co. Ltd, Kowa Pharmaceutical Co. Ltd, Ono Pharmaceutical Co. Ltd, Daiichi Sankyo Co. Ltd, Sanofi K.K., Mitsubishi‐Tanabe Pharma Corporation, Sanwa Kagaku Kenkyusho Co. Ltd, Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co. Ltd, Novo Nordisk Pharma Ltd, Bayer Yakuhin Ltd, Teijin Ohama Co. Ltd, Novartis Pharma K.K. and Nipro Corporation. H.W. has received grants from Kowa, Sanofi, Yakult, Eli Lilly, Novartis, Sanwa Kagaku Kenkyusho, Abbott Japan, Astellas Pharma, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Pfizer, Kissei Pharma, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma, Merck Sharp & Dohme, Novo Nordisk, Ono Pharmaceutical, Teijin, Taisho‐Toyama and Souiken; and has received personal fees from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Dainippon Sumitomo Pharma, Eli Lilly, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho, Kyowa Hakko Kirin, Terumo Corp, Fuji Film and Takeda. D.Y. has received consulting/lecture fees from Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co. Ltd and Takeda Pharmaceutical Company Limited, and grants from Arkray Inc., Novo Nordisk Pharma Ltd, Nippon Boehringer Ingelheim, Ono Pharmaceutical Co. Ltd, Taisho Pharmaceutical Co. Ltd, Takeda Pharmaceutical Company Limited and Terumo Corporation during the conduct of the study. K.N. has received lecture fees from Boehringer Ingelheim, Daiichi Sankyo, Mitsubishi Tanabe, Astellas, Bayer, MSD K.K., Takeda, Ono, Eli Lilly and Company and Otsuka; and research support from Boehringer Ingelheim, Teijin Pharma, Mitsubishi Tanabe, Asahi‐kasei, Terumo, Astellas, Bayer and Daiichi Sankyo. T.M. has received contributions from Boehringer Ingelheim, and honorariums from Boehringer Ingelheim and Eli Lilly and Company. J.W. receives speaker honoraria from Astra Zeneca, Daiichi Sankyo, Novartis, Novo Nordisk Pharma and Tanabe Mitsubishi, and receives grant support from Astellas, Baxter, Bayer, Chugai, Dainippon Sumitomo, Kyowa Kirin, Novo Nordisk Pharma, Ono, Otsuka, Tanabe Mitsubishi and Teijin.
Figures
References
-
- International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels, Belgium: International Diabetes Federation; 2021. https://www.diabetesatlas.org/en/. Accessed December 6, 2021.
-
- Juhaeri J, Gao S, Dai WS. Incidence rates of heart failure, stroke, and acute myocardial infarction among type 2 diabetic patients using insulin glargine and other insulin. Pharmacoepidemiol Drug Saf. 2009;18:497‐503. - PubMed
-
- McMurray JJ, Gerstein HC, Holman RR, et al. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843‐851. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

