Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective
- PMID: 35929488
- PMCID: PMC10087310
- DOI: 10.1111/dom.14831
Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective
Abstract
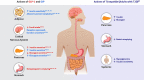
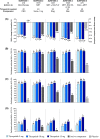
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide/glucagon-like peptide 1 (GLP-1) receptor agonist approved in the United States as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes and under investigation for use in chronic weight management, major adverse cardiovascular events and the management of other conditions, including heart failure with preserved ejection fraction and obesity and non-cirrhotic non-alcoholic steatohepatitis. The Phase 3 SURPASS 1-5 clinical trial programme was designed to assess efficacy and safety of once-weekly subcutaneously injected tirzepatide (5, 10 and 15 mg), as monotherapy or combination therapy, across a broad spectrum of people with type 2 diabetes. Use of tirzepatide in clinical studies was associated with marked reductions of glycated haemoglobin (-1.87 to -2.59%, -20 to -28 mmol/mol) and body weight (-6.2 to -12.9 kg), as well as reductions in parameters commonly associated with heightened cardiometabolic risk such as blood pressure, visceral adiposity and circulating triglycerides. In SUPRASS-2, these reductions were greater than with the GLP-1 receptor agonist semaglutide 1 mg. Tirzepatide was well tolerated, with a low risk of hypoglycaemia when used without insulin or insulin secretagogues and showed a generally similar safety profile to the GLP-1 receptor agonist class. Accordingly, evidence from these clinical trials suggests that tirzepatide offers a new opportunity for the effective lowering of glycated haemoglobin and body weight in adults with type 2 diabetes.
© 2022 Eli Lilly and Company and The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
CDB reports consulting fees from Eli Lilly and Company, Novo Nordisk, AstraZeneca, Boehringer‐Ingelheim and Abbott Diagnostics; and payment/honoraria from Eli Lilly and Company and Novo Nordisk. CB reports consulting fees from Abbott, BI, Eli Lilly and Company, and Merck; and payment/honoraria from BI, Intas, Merck and Novo. CW reports research funding from Eli Lilly and Company, Abbott, Corcept, Regeneron, Mylan and Novo Nordisk; participation on a Data Safety Monitoring Board for the NIH Artificial Pancreas Project; and is president of the Endocrine Society. AH, SEA and JP are employees and shareholders of Eli Lilly and Company.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

