Effects and potential mechanism of Ca2+/calmodulin‑dependent protein kinase II pathway inhibitor KN93 on the development of ovarian follicle
- PMID: 35929517
- PMCID: PMC9387563
- DOI: 10.3892/ijmm.2022.5177
Effects and potential mechanism of Ca2+/calmodulin‑dependent protein kinase II pathway inhibitor KN93 on the development of ovarian follicle
Abstract
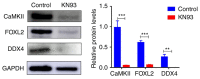
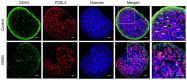
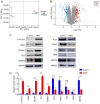
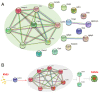
Adequate regulation of the speed of follicular development has been reported to prolong the reproductive life of the ovary. The aim of the present study was to assess the potential effects and mechanism of the Ca2+/calmodulin‑dependent protein kinase II (CaMKII) pathway on the development of ovarian follicle. In the present study, the expression of CaMKII was measured in the ovary of mice at different developmental stages by immunofluorescence, confirming that CaMKII has a role in follicular development. Subsequently, the 17.5 days post‑coitus (dpc) embryonic ovaries were collected and cultured with KN93 for 4 days in vitro. It was revealed that KN93 inhibited the development of follicles, where it reduced the expression levels of oocyte and granulosa cell markers DEAD‑box helicase 4 (DDX4) and forkhead box L2 (FOXL2). These results suggested that KN93 could delay follicular development. Proteomics technology was then used to find that 262 proteins of KN93 treated 17.5 dpc embryonic ovaries were significantly altered after in vitro culture. Bioinformatics analysis was used to analyze these altered proteins. In total, four important Kyoto Encyclopedia of Genes and Genome pathways, namely steroid biosynthesis, p53 signaling pathway and retinol metabolism and metabolic pathways, were particularly enriched. Further analysis revealed that the upregulated proteins NADP‑dependent steroid dehydrogenase‑like (Nsdhl), lanosterol synthase (Lss), farnesyl‑diphosphate farnesyltransferase 1 (Fdft1), cytochrome P450 family 51 family A member 1 (Cyp51a1), hydroxymethylglutaryl‑CoA synthase 1 (Hmgcs1), fatty acid synthase (Fasn) and dimethylallyltranstransferase (Fdps) were directly interacting with each other in the four enriched pathways. In summary, the potential mechanism of KN93 in slowing down follicular development most likely lies in its inhibitory effects on CaMKII, which upregulated the expression of Nsdhl, Lss, Fdft1, Cyp51a1, Hmgcs1, Fasn and Fdps. This downregulated the expression of oocyte and granulosa cell markers DDX4 and FOXL2 in the follicles, thereby delaying follicular development. Overall, these results provide novel insight into the potential mechanism by which KN93 and CaMKII can delay follicular development.
Keywords: KN93; calmodulin‑dependent protein kinase II; mouse; primary follicle; primordial follicle.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Granulosa cells from human primordial and primary follicles show differential global gene expression profiles.Hum Reprod. 2018 Apr 1;33(4):666-679. doi: 10.1093/humrep/dey011. Hum Reprod. 2018. PMID: 29506120
-
To explore the regulatory role of Wnt/P53/Caspase3 signal in mouse ovarian development based on LFQ proteomics.J Proteomics. 2023 Feb 10;272:104772. doi: 10.1016/j.jprot.2022.104772. Epub 2022 Nov 19. J Proteomics. 2023. PMID: 36414229
-
A novel three-dimensional culture system allows prolonged culture of functional human granulosa cells and mimics the ovarian environment.Tissue Eng Part A. 2010 Jun;16(6):2063-73. doi: 10.1089/ten.TEA.2009.0684. Tissue Eng Part A. 2010. PMID: 20109057
-
Non-esterified fatty acids in the ovary: friends or foes?Reprod Biol Endocrinol. 2020 Jun 6;18(1):60. doi: 10.1186/s12958-020-00617-9. Reprod Biol Endocrinol. 2020. PMID: 32505200 Free PMC article. Review.
-
Footmarks of innate immunity in the ovary and cytokeratin-positive cells as potential dendritic cells.Adv Anat Embryol Cell Biol. 2011;209:vii-99. doi: 10.1007/978-3-642-16077-6. Adv Anat Embryol Cell Biol. 2011. PMID: 21214088 Review.
Cited by
-
Epigallocatechin-3-gallate inhibits the formation of neutrophil extracellular traps and suppresses the migration and invasion of colon cancer cells by regulating STAT3/CXCL8 pathway.Mol Cell Biochem. 2023 Apr;478(4):887-898. doi: 10.1007/s11010-022-04550-w. Epub 2022 Sep 16. Mol Cell Biochem. 2023. PMID: 36112238
-
Discovery of FO-4-15, a novel 1,2,4-oxadiazole derivative, ameliorates cognitive impairments in 3×Tg mice by activating the mGluR1/CaMKIIα pathway.Acta Pharmacol Sin. 2025 Jan;46(1):66-80. doi: 10.1038/s41401-024-01362-0. Epub 2024 Aug 16. Acta Pharmacol Sin. 2025. PMID: 39152295
-
Transcriptome and proteome profile analysis of the regulation of chicken ovarian development.Poult Sci. 2025 Aug;104(8):105384. doi: 10.1016/j.psj.2025.105384. Epub 2025 May 31. Poult Sci. 2025. PMID: 40466268 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

