Gene therapy targeting the blood-brain barrier improves neurological symptoms in a model of genetic MCT8 deficiency
- PMID: 35929549
- PMCID: PMC9762946
- DOI: 10.1093/brain/awac243
Gene therapy targeting the blood-brain barrier improves neurological symptoms in a model of genetic MCT8 deficiency
Abstract
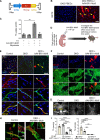
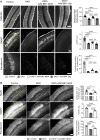
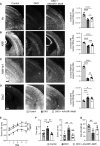
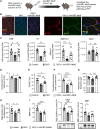
A genetic deficiency of the solute carrier monocarboxylate transporter 8 (MCT8), termed Allan-Herndon-Dudley syndrome, is an important cause of X-linked intellectual and motor disability. MCT8 transports thyroid hormones across cell membranes. While thyroid hormone analogues improve peripheral changes of MCT8 deficiency, no treatment of the neurological symptoms is available so far. Therefore, we tested a gene replacement therapy in Mct8- and Oatp1c1-deficient mice as a well-established model of the disease. Here, we report that targeting brain endothelial cells for Mct8 expression by intravenously injecting the vector AAV-BR1-Mct8 increased tri-iodothyronine (T3) levels in the brain and ameliorated morphological and functional parameters associated with the disease. Importantly, the therapy resulted in a long-lasting improvement in motor coordination. Thus, the data support the concept that MCT8 mediates the transport of thyroid hormones into the brain and indicate that a readily accessible vascular target can help overcome the consequences of the severe disability associated with MCT8 deficiency.
Keywords: cretinism; hypothyroidism; myelination; thyroxine; tri-iodothyronine.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

Similar articles
-
Toward a treatment for thyroid hormone transporter MCT8 deficiency - achievements and challenges.Eur Thyroid J. 2024 Nov 20;13(6):e240286. doi: 10.1530/ETJ-24-0286. Print 2024 Dec 1. Eur Thyroid J. 2024. PMID: 39485732 Free PMC article. Review.
-
TRIAC Treatment Improves Impaired Brain Network Function and White Matter Loss in Thyroid Hormone Transporter Mct8/Oatp1c1 Deficient Mice.Int J Mol Sci. 2022 Dec 8;23(24):15547. doi: 10.3390/ijms232415547. Int J Mol Sci. 2022. PMID: 36555189 Free PMC article.
-
Brain Gene Expression in Systemic Hypothyroidism and Mouse Models of MCT8 Deficiency: The Mct8-Oatp1c1-Dio2 Triad.Thyroid. 2021 Jun;31(6):985-993. doi: 10.1089/thy.2020.0649. Epub 2021 Mar 18. Thyroid. 2021. PMID: 33307956
-
Neurovascular unit disruption and blood-brain barrier leakage in MCT8 deficiency.Fluids Barriers CNS. 2023 Nov 3;20(1):79. doi: 10.1186/s12987-023-00481-w. Fluids Barriers CNS. 2023. PMID: 37924081 Free PMC article.
-
Thyroid hormone transporters--functions and clinical implications.Nat Rev Endocrinol. 2015 Jul;11(7):406-17. doi: 10.1038/nrendo.2015.66. Epub 2015 May 5. Nat Rev Endocrinol. 2015. PMID: 25942657 Review.
Cited by
-
Antigen recognition detains CD8+ T cells at the blood-brain barrier and contributes to its breakdown.Nat Commun. 2023 May 30;14(1):3106. doi: 10.1038/s41467-023-38703-2. Nat Commun. 2023. PMID: 37253744 Free PMC article.
-
Toward a treatment for thyroid hormone transporter MCT8 deficiency - achievements and challenges.Eur Thyroid J. 2024 Nov 20;13(6):e240286. doi: 10.1530/ETJ-24-0286. Print 2024 Dec 1. Eur Thyroid J. 2024. PMID: 39485732 Free PMC article. Review.
-
TRIAC Treatment Improves Impaired Brain Network Function and White Matter Loss in Thyroid Hormone Transporter Mct8/Oatp1c1 Deficient Mice.Int J Mol Sci. 2022 Dec 8;23(24):15547. doi: 10.3390/ijms232415547. Int J Mol Sci. 2022. PMID: 36555189 Free PMC article.
-
Thyroid Hormone Transporters in Pregnancy and Fetal Development.Int J Mol Sci. 2022 Dec 1;23(23):15113. doi: 10.3390/ijms232315113. Int J Mol Sci. 2022. PMID: 36499435 Free PMC article. Review.
-
Functional gene delivery to and across brain vasculature of systemic AAVs with endothelial-specific tropism in rodents and broad tropism in primates.Nat Commun. 2023 Jun 8;14(1):3345. doi: 10.1038/s41467-023-38582-7. Nat Commun. 2023. PMID: 37291094 Free PMC article.
References
-
- Wood-Allum CA, Shaw PJ. Thyroid disease and the nervous system. Handb Clin Neurol. 2014;120:703–735. - PubMed
-
- Halpern JP, Boyages SC, Maberly GF, Collins JK, Eastman CJ, Morris JG. The neurology of endemic cretinism. A study of two endemias. Brain. 1991;114(Pt 2):825–841. - PubMed
-
- Tan ZS, Vasan RS. Thyroid function and Alzheimer’s disease. J Alzheimers Dis. 2009;16:503–507. - PubMed
-
- Bernal J, Guadano-Ferraz A, Morte B. Thyroid hormone transporters—Functions and clinical implications. Review. Nat Rev Endocrinol. 2015;11:406–417. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

