Biocatalysis: A smart and green tool for the preparation of chiral drugs
- PMID: 35929567
- PMCID: PMC9805200
- DOI: 10.1002/chir.23498
Biocatalysis: A smart and green tool for the preparation of chiral drugs
Abstract
Over the last decades, biocatalysis has achieved growing interest thanks to its potential to enable high efficiency, high yield, and eco-friendly processes aimed at the production of pharmacologically relevant compounds. Particularly, biocatalysis proved an effective and potent tool in the preparation of chiral molecules, and the recent innovations of biotechnologies and nanotechnologies open up a new era of further developments in this field. Different strategies are now available for the synthesis of chiral drugs and their intermediates. Enzymes are green tools that offer several advantages, associated both to catalysis and environmentally friendly reactants. Specifically, the use of enzymes isolated from biological sources or of whole-cell represents a valuable approach to obtain pharmaceutical products. The sustainability, the higher efficiency, and cost-effectiveness of biocatalytic reactions result in improved performance and properties that can be translated from academia to industry. In this review, we focus on biocatalytic approaches for synthesizing chiral drugs or their intermediates. Aiming to unveil the potentialities of biocatalysis systems, we discuss different examples of innovative biocatalytic approaches and their applications in the pharmaceutical industry.
Keywords: biocatalysis; chiral resolution; enantioselective synthesis; green chemistry; homochiral drugs.
© 2022 The Authors. Chirality published by Wiley Periodicals LLC.
Figures


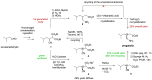
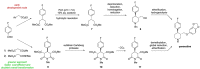
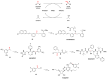
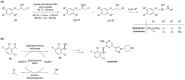
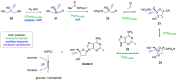
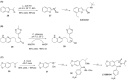
References
-
- Bell EL, Finnigan W, France SP, et al. Biocatalysis. Nat Rev Methods Primers. 2021;1(1):1‐21. doi:10.1038/s43586-021-00044-z - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

