Characterization of TR-107, a novel chemical activator of the human mitochondrial protease ClpP
- PMID: 35929764
- PMCID: PMC9354705
- DOI: 10.1002/prp2.993
Characterization of TR-107, a novel chemical activator of the human mitochondrial protease ClpP
Abstract
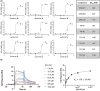
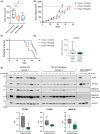
We recently described the identification of a new class of small-molecule activators of the mitochondrial protease ClpP. These compounds synthesized by Madera Therapeutics showed increased potency of cancer growth inhibition over the related compound ONC201. In this study, we describe chemical optimization and characterization of the next generation of highly potent and selective small-molecule ClpP activators (TR compounds) and demonstrate their efficacy against breast cancer models in vitro and in vivo. We selected one compound (TR-107) with excellent potency, specificity, and drug-like properties for further evaluation. TR-107 showed ClpP-dependent growth inhibition in the low nanomolar range that was equipotent to paclitaxel in triple-negative breast cancer (TNBC) cell models. TR-107 also reduced specific mitochondrial proteins, including OXPHOS and TCA cycle components, in a time-, dose-, and ClpP-dependent manner. Seahorse XF analysis and glucose deprivation experiments confirmed the inactivation of OXPHOS and increased dependence on glycolysis following TR-107 exposure. The pharmacokinetic properties of TR-107 were compared with other known ClpP activators including ONC201 and ONC212. TR-107 displayed excellent exposure and serum t1/2 after oral administration. Using human TNBC MDA-MB-231 xenografts, the antitumor response to TR-107 was investigated. Oral administration of TR-107 resulted in a reduction in tumor volume and extension of survival in the treated compared with vehicle control mice. ClpP activation in vivo was validated by immunoblotting for TFAM and other mitochondrial proteins. In summary, we describe the identification of highly potent new ClpP agonists with improved efficacy against TNBC, through targeted inactivation of OXPHOS and disruption of mitochondrial metabolism.
Keywords: agonist; cell proliferation; mitochondria; oxidative phosphorylation; protease; small molecule; triple-negative breast cancer.
© 2022 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Figures



Similar articles
-
Multi-omics analyses reveal ClpP activators disrupt essential mitochondrial pathways in triple-negative breast cancer.Front Pharmacol. 2023 Mar 31;14:1136317. doi: 10.3389/fphar.2023.1136317. eCollection 2023. Front Pharmacol. 2023. PMID: 37063293 Free PMC article.
-
Dose Optimization of ClpP Agonists Using an In Vitro Microfluidic Perfusion Platform and In Silico Pharmacokinetic-Pharmacodynamic Modeling.AAPS J. 2025 Jun 13;27(4):109. doi: 10.1208/s12248-025-01088-9. AAPS J. 2025. PMID: 40514581
-
Metabolic Targeting of Oxidative Phosphorylation Enhances Chemosensitivity in Triple-Negative Breast Cancer via a Synergistic Nanomedicine.Theranostics. 2025 Jun 23;15(15):7607-7626. doi: 10.7150/thno.116250. eCollection 2025. Theranostics. 2025. PMID: 40756342 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
A review of current therapeutics targeting the mitochondrial protease ClpP in diffuse midline glioma, H3 K27-altered.Neuro Oncol. 2024 May 3;26(Supplement_2):S136-S154. doi: 10.1093/neuonc/noad144. Neuro Oncol. 2024. PMID: 37589388 Free PMC article. Review.
-
Discovery of CLPP-1071 as an Exceptionally Potent and Orally Efficacious Human ClpP Activator with Strong In Vivo Antitumor Activity.J Med Chem. 2024 Dec 12;67(23):21009-21029. doi: 10.1021/acs.jmedchem.4c01605. Epub 2024 Nov 22. J Med Chem. 2024. PMID: 39574384 Free PMC article.
-
Multi-omics analyses reveal ClpP activators disrupt essential mitochondrial pathways in triple-negative breast cancer.Front Pharmacol. 2023 Mar 31;14:1136317. doi: 10.3389/fphar.2023.1136317. eCollection 2023. Front Pharmacol. 2023. PMID: 37063293 Free PMC article.
-
TR-107, an Agonist of Caseinolytic Peptidase Proteolytic Subunit, Disrupts Mitochondrial Metabolism and Inhibits the Growth of Human Colorectal Cancer Cells.Mol Cancer Ther. 2024 Dec 3;23(12):1761-1778. doi: 10.1158/1535-7163.MCT-24-0170. Mol Cancer Ther. 2024. PMID: 39233476 Free PMC article.
-
TUFM in health and disease: exploring its multifaceted roles.Front Immunol. 2024 May 29;15:1424385. doi: 10.3389/fimmu.2024.1424385. eCollection 2024. Front Immunol. 2024. PMID: 38868764 Free PMC article. Review.
References
-
- Villacampa G, Tolosa P, Salvador F, et al. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first‐line metastatic triple‐negative breast cancer: a systematic review and meta‐analysis. Cancer Treat Rev. 2022;104:102352. doi: 10.1016/j.ctrv.2022.102352 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

