β-cell dynamics in type 2 diabetes and in dietary and exercise interventions
- PMID: 35929791
- PMCID: PMC9710517
- DOI: 10.1093/jmcb/mjac046
β-cell dynamics in type 2 diabetes and in dietary and exercise interventions
Abstract
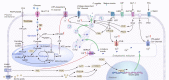
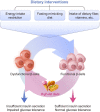
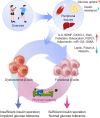
Pancreatic β-cell dysfunction and insulin resistance are two of the major causes of type 2 diabetes (T2D). Recent clinical and experimental studies have suggested that the functional capacity of β-cells, particularly in the first phase of insulin secretion, is a primary contributor to the progression of T2D and its associated complications. Pancreatic β-cells undergo dynamic compensation and decompensation processes during the development of T2D, in which metabolic stresses such as endoplasmic reticulum stress, oxidative stress, and inflammatory signals are key regulators of β-cell dynamics. Dietary and exercise interventions have been shown to be effective approaches for the treatment of obesity and T2D, especially in the early stages. Whilst the targeted tissues and underlying mechanisms of dietary and exercise interventions remain somewhat vague, accumulating evidence has implicated the improvement of β-cell functional capacity. In this review, we summarize recent advances in the understanding of the dynamic adaptations of β-cell function in T2D progression and clarify the effects and mechanisms of dietary and exercise interventions on β-cell dysfunction in T2D. This review provides molecular insights into the therapeutic effects of dietary and exercise interventions on T2D, and more importantly, it paves the way for future research on the related underlying mechanisms for developing precision prevention and treatment of T2D.
Keywords: dietary intervention; exercise intervention; pancreatic β-cell; type 2 diabetes.
© The Author(s) (2022). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures

Similar articles
-
Transcriptional control of pancreatic β-cell identity and plasticity during the pathogenesis of type 2 diabetes.J Genet Genomics. 2022 Apr;49(4):316-328. doi: 10.1016/j.jgg.2022.03.002. Epub 2022 Mar 12. J Genet Genomics. 2022. PMID: 35292418 Review.
-
Functional high-intensity training improves pancreatic β-cell function in adults with type 2 diabetes.Am J Physiol Endocrinol Metab. 2017 Sep 1;313(3):E314-E320. doi: 10.1152/ajpendo.00407.2016. Epub 2017 May 16. Am J Physiol Endocrinol Metab. 2017. PMID: 28512155 Free PMC article.
-
Molecular mechanism of islet β-cell functional alternations during type 2 diabetes.Yi Chuan. 2022 Oct 20;44(10):840-852. doi: 10.16288/j.yczz.22-265. Yi Chuan. 2022. PMID: 36384722 Review.
-
Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes.J Mol Endocrinol. 2016 Feb;56(2):R33-54. doi: 10.1530/JME-15-0232. Epub 2015 Nov 17. J Mol Endocrinol. 2016. PMID: 26576641 Review.
-
β-Cell compensation concomitant with adaptive endoplasmic reticulum stress and β-cell neogenesis in a diet-induced type 2 diabetes model.Appl Physiol Nutr Metab. 2019 Dec;44(12):1355-1366. doi: 10.1139/apnm-2019-0144. Epub 2019 May 13. Appl Physiol Nutr Metab. 2019. PMID: 31082326
Cited by
-
The Levels of Phoenixin-14 and Phoenixin-20 in Patients with Type 2 Diabetes Mellitus.Endocr Metab Immune Disord Drug Targets. 2024;24(11):1315-1322. doi: 10.2174/0118715303267256231210060250. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 38213155
-
Vitamin D Alleviates Type 2 Diabetes Mellitus by Mitigating Oxidative Stress-Induced Pancreatic β-Cell Impairment.Exp Clin Endocrinol Diabetes. 2023 Dec;131(12):656-666. doi: 10.1055/a-2191-9969. Epub 2023 Nov 7. Exp Clin Endocrinol Diabetes. 2023. PMID: 37935388 Free PMC article.
-
Effect of long-term negative energy on appetite hormone levels in individuals with prediabetes and diabetes.Rev Assoc Med Bras (1992). 2025 Jan 10;71(1):e20240897. doi: 10.1590/1806-9282.20240897. eCollection 2025. Rev Assoc Med Bras (1992). 2025. PMID: 39813443 Free PMC article.
-
Lycopene ameliorates diabetes-induced pancreatic, hepatic, and renal damage by modulating the JAK/STAT/SOCS signaling pathway in rats.Iran J Basic Med Sci. 2025;28(4):461-468. doi: 10.22038/ijbms.2025.79979.17326. Iran J Basic Med Sci. 2025. PMID: 39968090 Free PMC article.
-
Recent insights of obesity-induced gut and adipose tissue dysbiosis in type 2 diabetes.Front Mol Biosci. 2023 Sep 28;10:1224982. doi: 10.3389/fmolb.2023.1224982. eCollection 2023. Front Mol Biosci. 2023. PMID: 37842639 Free PMC article. Review.
References
-
- Al-Aubaidy H.A., Jelinek H.F. (2011). Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur. J. Endocrinol. 164, 899–904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

