Pericentrin interacts with Kinesin-1 to drive centriole motility
- PMID: 35929834
- PMCID: PMC9361567
- DOI: 10.1083/jcb.202112097
Pericentrin interacts with Kinesin-1 to drive centriole motility
Abstract
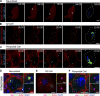
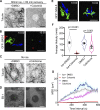
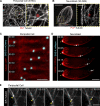
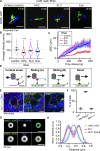
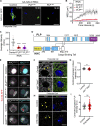
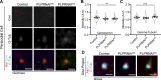
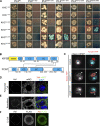
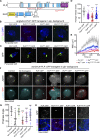
Centrosome positioning is essential for their function. Typically, centrosomes are transported to various cellular locations through the interaction of centrosomal microtubules (MTs) with motor proteins anchored at the cortex or the nuclear surface. However, it remains unknown how centrioles migrate in cellular contexts in which they do not nucleate MTs. Here, we demonstrate that during interphase, inactive centrioles move directly along the interphase MT network as Kinesin-1 cargo. We identify Pericentrin-Like-Protein (PLP) as a novel Kinesin-1 interacting molecule essential for centriole motility. In vitro assays show that PLP directly interacts with the cargo binding domain of Kinesin-1, allowing PLP to migrate on MTs. Binding assays using purified proteins revealed that relief of Kinesin-1 autoinhibition is critical for its interaction with PLP. Finally, our studies of neural stem cell asymmetric divisions in the Drosophila brain show that the PLP-Kinesin-1 interaction is essential for the timely separation of centrioles, the asymmetry of centrosome activity, and the age-dependent centrosome inheritance.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Figures






Similar articles
-
Positioning centrioles and centrosomes.J Cell Biol. 2024 Apr 1;223(4):e202311140. doi: 10.1083/jcb.202311140. Epub 2024 Mar 21. J Cell Biol. 2024. PMID: 38512059 Free PMC article. Review.
-
Drosophila PLP assembles pericentriolar clouds that promote centriole stability, cohesion and MT nucleation.PLoS Genet. 2018 Feb 9;14(2):e1007198. doi: 10.1371/journal.pgen.1007198. eCollection 2018 Feb. PLoS Genet. 2018. PMID: 29425198 Free PMC article.
-
Sperm Head-Tail Linkage Requires Restriction of Pericentriolar Material to the Proximal Centriole End.Dev Cell. 2020 Apr 6;53(1):86-101.e7. doi: 10.1016/j.devcel.2020.02.006. Epub 2020 Mar 12. Dev Cell. 2020. PMID: 32169161 Free PMC article.
-
PLP inhibits the activity of interphase centrosomes to ensure their proper segregation in stem cells.J Cell Biol. 2013 Sep 30;202(7):1013-22. doi: 10.1083/jcb.201303141. J Cell Biol. 2013. PMID: 24081489 Free PMC article.
-
Centrosome function: sometimes less is more.Traffic. 2009 May;10(5):472-81. doi: 10.1111/j.1600-0854.2009.00880.x. Epub 2009 Jan 24. Traffic. 2009. PMID: 19192251 Review.
Cited by
-
Positioning centrioles and centrosomes.J Cell Biol. 2024 Apr 1;223(4):e202311140. doi: 10.1083/jcb.202311140. Epub 2024 Mar 21. J Cell Biol. 2024. PMID: 38512059 Free PMC article. Review.
-
A potential patient stratification biomarker for Parkinson´s disease based on LRRK2 kinase-mediated centrosomal alterations in peripheral blood-derived cells.NPJ Parkinsons Dis. 2024 Jan 8;10(1):12. doi: 10.1038/s41531-023-00624-8. NPJ Parkinsons Dis. 2024. PMID: 38191886 Free PMC article.
-
Rab11 endosomes and Pericentrin coordinate centrosome movement during pre-abscission in vivo.Life Sci Alliance. 2022 Mar 18;5(7):e202201362. doi: 10.26508/lsa.202201362. Print 2022 Jul. Life Sci Alliance. 2022. PMID: 35304423 Free PMC article.
-
Microtubule-driven cell shape changes and actomyosin flow synergize to position the centrosome.J Cell Biol. 2025 Jul 7;224(7):e202405126. doi: 10.1083/jcb.202405126. Epub 2025 Apr 17. J Cell Biol. 2025. PMID: 40243666
-
Asymmetry of centrosomes in Drosophila neural stem cells requires protein phosphatase 4.Mol Biol Cell. 2025 May 1;36(5):ar58. doi: 10.1091/mbc.E25-01-0021. Epub 2025 Mar 12. Mol Biol Cell. 2025. PMID: 40072519
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

