Aging compromises oligodendrocyte precursor cell maturation and efficient remyelination in the monkey brain
- PMID: 35930094
- PMCID: PMC9886778
- DOI: 10.1007/s11357-022-00621-4
Aging compromises oligodendrocyte precursor cell maturation and efficient remyelination in the monkey brain
Abstract
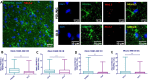
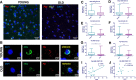
Age-associated cognitive decline is common among otherwise healthy elderly people, even in the absence of Alzheimer's disease and neuron loss. Instead, white matter loss and myelin damage are strongly associated with cognitive decline. Myelin is subject to lifelong oxidative stress that damages the myelin sheath, which is repaired by cells of the oligodendrocyte lineage. This process is mediated by oligodendrocyte precursor cells (OPCs) that sense the damage and respond by proliferating locally and migrating to the region, where they differentiate into mature myelinating oligodendrocytes. In aging, extensive myelin damage, in combination with inefficient remyelination, leads to chronically damaged myelin and loss of efficient neuronal conduction. This study used the rhesus monkey model of normal aging to examine how myelin regeneration capacity is affected by age. Results show that older subjects have reduced numbers of new BCAS1 + myelinating oligodendrocytes, which are newly formed cells, and that this reduction is associated with poorer cognitive performance. Interestingly, this does not result from limited proliferation of progenitor OPCs. Instead, the transcription factor NKX2.2, which regulates OPCs differentiation, is significantly decreased in aged OPCs. This suggests that these OPCs have a diminished potential for differentiation into mature oligodendrocytes. In addition, mature oligodendrocytes have reduced RNA expression of two essential myelin protein markers, MBP and PLP. These data collectively suggest that in the normal aging brain, there is a reduction in regenerative OPCs as well as myelin production that impairs the capacity for remyelination.
Keywords: Aging; Myelination; Oligodendrocyte; RNAscope.
© 2022. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

