Cost-Utility Analysis of Pharmacogenetic Testing Based on CYP2C19 or CYP2D6 in Major Depressive Disorder: Assessing the Drivers of Different Cost-Effectiveness Levels from an Italian Societal Perspective
- PMID: 35930170
- PMCID: PMC9427916
- DOI: 10.1007/s40261-022-01182-2
Cost-Utility Analysis of Pharmacogenetic Testing Based on CYP2C19 or CYP2D6 in Major Depressive Disorder: Assessing the Drivers of Different Cost-Effectiveness Levels from an Italian Societal Perspective
Abstract
Background and objectives: Major depressive disorder (MDD) is a common and severe psychiatric disorder that has enormous economical and societal costs. As pharmacogenetics is one of the key tools of precision psychiatry, we analyze the cost-utility of test screening of CYP2C19 and CYP2D6 for patients suffering from major depressive disorder (MDD) and try to understand the main drivers that influence the cost-utility.
Methods: We developed two pharmacoeconomic nonhomogeneous Markov models to test the cost-utility, from an Italian societal perspective, of pharmacogenetic testing genetic to characterize the metabolizing profiles of cytochrome P450 (CYP) 2C19 and CYP2D6 in a hypothetical case study of patients suffering from major depressive disorder (MDD). The model considers different scenarios of adjustment of antidepressant treatment according to the patient's metabolizing profile or treatment over a period of 18 weeks. The uncertainty of model parameters is tested through both a probabilistic sensitivity analysis and a one-way deterministic sensitivity analysis, and these results are used in a post-hoc analysis to understand the main drivers of three alternative cost-effectiveness levels ("poor," "standard," and "high"). These drivers are first evaluated from an exploratory multidimensional perspective and next from a predictive perspective as the probability that a patient belongs to a specific cost-effectiveness level is estimated on the basis of a restricted set of parameters used in the original pharmacoeconomic model.
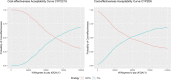
Results: The models for CYP2C19 and CYP2D6 indicate that screening has an incremental cost-effectiveness ratio of 60,000€ and 47,000€ per quality-adjusted life year (QALY), respectively. The probabilistic sensitivity analysis shows that the treatments are cost-effective for a 75,000€ willingness to pay (WTP) threshold in 58% and 63% of the Monte Carlo replications, respectively. The post-hoc analysis highlights the factors that allow us to clearly discriminates poor cost-effectiveness from high cost-effectiveness scenarios and demonstrates that it is possible to predict with reasonable accuracy the cost-effectiveness of a genetic test and the associated therapeutic pattern.
Conclusions: Our findings suggest that screenings for both CYP2C19 and CYP2D6 enzymes for patients with MDD are cost-effective for a WTP threshold of 75,000€ per QALY, and provide relevant suggestions about the most important aspects to be further explored in clinical studies aimed at addressing the cost-effectiveness of genetic testing for patients diagnosed with MDD.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest.
Figures





References
-
- Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

