Efficacy and safety of nafamostat mesilate anticoagulation in blood purification treatment of critically ill patients: a systematic review and meta-analysis
- PMID: 35930302
- PMCID: PMC9359194
- DOI: 10.1080/0886022X.2022.2105233
Efficacy and safety of nafamostat mesilate anticoagulation in blood purification treatment of critically ill patients: a systematic review and meta-analysis
Abstract
Background: Nafamostat mesilate (NM), a broad-spectrum and potent serine protease inhibitor, can be used as an anticoagulant during extracorporeal circulation, as well as a promising drug effective against coronavirus disease 2019 (COVID-19). We conducted a systematic meta-analysis to evaluate the safety and efficacy of NM administration in critically ill patients who underwent blood purification therapy (BPT).
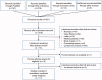
Methods: The Cochrane Library, Web of Science and PubMed were comprehensively searched from inception to August 20, 2021, for potential studies.
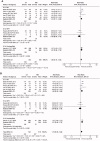
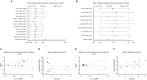
Results: Four randomized controlled trials (RCTs) and seven observational studies with 2723 patients met the inclusion criteria. The meta-analysis demonstrated that conventional therapy (CT) significantly increased hospital mortality compared with NM administration (RR = 1.25, p = 0.0007). In subgroup analyses, the in-hospital mortality of the NM group was significantly lower than that of the anticoagulant-free (NA) group (RR = 1.31, p = 0.002). The CT interventions markedly elevated the risk ratio of bleeding complications by 45% (RR = 1.45, p = 0.010) compared with NM interventions. In another subgroup analysis, NM used exhibited a significantly lower risk of bleeding complications than those of the low-molecular-weight heparin (LMWH) used (RR = 4.58, p = 0.020). The filter lifespan was decreased significantly (MD = -10.59, p < 0.0001) in the NA groups compared with the NM groups. Due to the poor quality of the included RCTs, these results should be interpreted with caution.
Conclusion: Given the better survival outcomes, lower risk of bleeding, NM anticoagulation seems to be a safe and efficient approach for BPT patients and could yield a favorable filter lifespan. More multi-center RCTs with large samples are required for further validation of this study.
Keywords: COVID-19; Nafamostat mesilate; bleeding complication; blood purification; mortality.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





Similar articles
-
Nafamostat Mesilate as an Anticoagulant During Continuous Renal Replacement Therapy in Patients With High Bleeding Risk: A Randomized Clinical Trial.Medicine (Baltimore). 2015 Dec;94(52):e2392. doi: 10.1097/MD.0000000000002392. Medicine (Baltimore). 2015. PMID: 26717390 Free PMC article. Clinical Trial.
-
Nafamostat mesilate for anticoagulation in continuous renal replacement therapy.Int J Artif Organs. 2013 Mar;36(3):208-16. doi: 10.5301/IJAO.5000191. Epub 2013 Feb 13. Int J Artif Organs. 2013. PMID: 23404639
-
Multi-centre, three arm, randomized controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS-CoV-2 infection: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Aug 17;21(1):724. doi: 10.1186/s13063-020-04645-z. Trials. 2020. PMID: 32807241 Free PMC article.
-
Anticoagulation with nafamostat mesilate during extracorporeal life support.Int J Cardiol. 2022 Nov 1;366:71-79. doi: 10.1016/j.ijcard.2022.07.022. Epub 2022 Jul 16. Int J Cardiol. 2022. PMID: 35850387 Review.
-
Pharmacological interventions for preventing clotting of extracorporeal circuits during continuous renal replacement therapy.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD012467. doi: 10.1002/14651858.CD012467.pub3. Cochrane Database Syst Rev. 2020. PMID: 33314078 Free PMC article.
Cited by
-
Comparison of regional citrate anticoagulation and nafamostat mesylate anticoagulation during plasma exchange for children at high bleeding risk: a retrospective study.Ital J Pediatr. 2025 Apr 12;51(1):114. doi: 10.1186/s13052-025-01954-4. Ital J Pediatr. 2025. PMID: 40221768 Free PMC article.
-
How to safeguard the continuous renal replacement therapy circuit: a narrative review.Front Med (Lausanne). 2024 Aug 21;11:1442065. doi: 10.3389/fmed.2024.1442065. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39234046 Free PMC article. Review.
-
Efficacy and safety of Nafamostat mesylate in patients with end-stage renal failure.World J Clin Cases. 2024 Jan 6;12(1):68-75. doi: 10.12998/wjcc.v12.i1.68. World J Clin Cases. 2024. PMID: 38292627 Free PMC article.
-
Dose of nafamostat mesylate during continuous kidney replacement therapy in critically ill patients: a two-centre observational study.BMC Nephrol. 2024 Feb 26;25(1):69. doi: 10.1186/s12882-024-03506-0. BMC Nephrol. 2024. PMID: 38408970 Free PMC article.
-
Filter Lifespan, Treatment Effect, and Influencing Factors of Continuous Renal Replacement Therapy for Severe Burn Patients.J Burn Care Res. 2024 May 6;45(3):764-770. doi: 10.1093/jbcr/irad196. J Burn Care Res. 2024. PMID: 38113522 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
