Near-infrared spectroscopy estimation of combined skeletal muscle oxidative capacity and O2 diffusion capacity in humans
- PMID: 35930524
- PMCID: PMC9481735
- DOI: 10.1113/JP283267
Near-infrared spectroscopy estimation of combined skeletal muscle oxidative capacity and O2 diffusion capacity in humans
Abstract
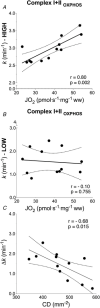
The final steps of the O2 cascade during exercise depend on the product of the microvascular-to-intramyocyte difference and muscle O2 diffusing capacity ( ). Non-invasive methods to determine in humans are currently unavailable. Muscle oxygen uptake (m ) recovery rate constant (k), measured by near-infrared spectroscopy (NIRS) using intermittent arterial occlusions, is associated with muscle oxidative capacity in vivo. We reasoned that k would be limited by when muscle oxygenation is low (kLOW ), and hypothesized that: (i) k in well oxygenated muscle (kHIGH ) is associated with maximal O2 flux in fibre bundles; and (ii) ∆k (kHIGH - kLOW ) is associated with capillary density (CD). Vastus lateralis k was measured in 12 participants using NIRS after moderate exercise. The timing and duration of arterial occlusions were manipulated to maintain tissue saturation index within a 10% range either below (LOW) or above (HIGH) half-maximal desaturation, assessed during sustained arterial occlusion. Maximal O2 flux in phosphorylating state was 37.7 ± 10.6 pmol s-1 mg-1 (∼5.8 ml min-1 100 g-1 ). CD ranged 348 to 586 mm-2 . kHIGH was greater than kLOW (3.15 ± 0.45 vs. 1.56 ± 0.79 min-1 , P < 0.001). Maximal O2 flux was correlated with kHIGH (r = 0.80, P = 0.002) but not kLOW (r = -0.10, P = 0.755). Δk ranged -0.26 to -2.55 min-1 , and correlated with CD (r = -0.68, P = 0.015). m k reflects muscle oxidative capacity only in well oxygenated muscle. ∆k, the difference in k between well and poorly oxygenated muscle, was associated with CD, a mediator of . Assessment of muscle k and ∆k using NIRS provides a non-invasive window on muscle oxidative and O2 diffusing capacity. KEY POINTS: We determined post-exercise recovery kinetics of quadriceps muscle oxygen uptake (m ) measured by near-infrared spectroscopy (NIRS) in humans under conditions of both non-limiting (HIGH) and limiting (LOW) O2 availability, for comparison with biopsy variables. The m recovery rate constant in HIGH O2 availability was hypothesized to reflect muscle oxidative capacity (kHIGH ) and the difference in k between HIGH and LOW O2 availability (∆k) was hypothesized to reflect muscle O2 diffusing capacity. kHIGH was correlated with phosphorylating oxidative capacity of permeabilized muscle fibre bundles (r = 0.80). ∆k was negatively correlated with capillary density (r = -0.68) of biopsy samples. NIRS provides non-invasive means of assessing both muscle oxidative and oxygen diffusing capacity in vivo.
Keywords: biopsy; capillary density; mitochondria; recovery kinetics; skeletal muscle.
© 2022 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures
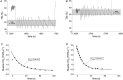
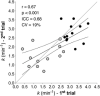
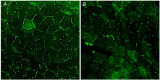
Comment in
-
Crossing the final frontier: oxygen transport at the blood-myocyte boundary.J Physiol. 2022 Oct;600(20):4385-4386. doi: 10.1113/JP283659. Epub 2022 Sep 22. J Physiol. 2022. PMID: 36083226 No abstract available.
-
Using near-infrared spectroscopy to explore cardiovascular function and muscle oxidative properties within people with Parkinson's disease.J Physiol. 2022 Nov;600(22):4807-4809. doi: 10.1113/JP283759. Epub 2022 Oct 17. J Physiol. 2022. PMID: 36183240 No abstract available.
-
Muscle O2 diffusion capacity by NIRS: A new approach in the air.J Physiol. 2022 Dec;600(23):5163-5164. doi: 10.1113/JP283882. Epub 2022 Oct 17. J Physiol. 2022. PMID: 36205221 No abstract available.
-
Reply to the letter from Manferdelli et al.: 'Muscle O2 diffusion capacity by NIRS: a new approach in the air'.J Physiol. 2022 Dec;600(23):5165-5166. doi: 10.1113/JP283919. Epub 2022 Nov 19. J Physiol. 2022. PMID: 36335427 No abstract available.
-
Shedding light on the assessment of skeletal muscle capillarization using near-infrared spectroscopy: future directions and applications.J Physiol. 2023 Jan;601(2):253-254. doi: 10.1113/JP284032. Epub 2022 Dec 18. J Physiol. 2023. PMID: 36495289 No abstract available.
References
-
- Adami, A. , & Rossiter, H. B. (2018). Principles, insights, and potential pitfalls of the noninvasive determination of muscle oxidative capacity by near‐infrared spectroscopy. Journal of Applied Physiology, 124(1), 245–248. - PubMed
-
- Beaver, W. L. , Wasserman, K. , & Whipp, B. J. (1986). A new method for detecting anaerobic threshold by gas exchange. Journal of Applied Physiology, 60(6), 2020–2027. - PubMed
-
- Bebout, D. E. , Hogan, M. C. , Hempleman, S. C. , & Wagner, P. D. (1993). Effects of training and immobilization on VO2 and DO2 in dog gastrocnemius muscle in situ. Journal of Applied Physiology, 74(4), 1697–1703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

