FXR deficiency in hepatocytes disrupts the bile acid homeostasis and inhibits autophagy to promote liver injury in Schistosoma japonicum-infected mice
- PMID: 35930537
- PMCID: PMC9355238
- DOI: 10.1371/journal.pntd.0010651
FXR deficiency in hepatocytes disrupts the bile acid homeostasis and inhibits autophagy to promote liver injury in Schistosoma japonicum-infected mice
Abstract
Background: Schistosomiasis, with 250 million people affected, is characterized by its serious hepatic inflammatory response and fibrosis formation, which could lead to dangerous complications, such as portal hypertension, splenomegaly and even ascites. But until now, the pathogenesis of schistosomiasis remains largely unknown. Farnesoid X Receptor (FXR), a bile acid-activated nuclear transcription factor mainly expresses in hepatocytes in the liver, can regulate liver diseases by controlling bile acid metabolism.
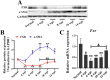
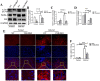
Methodology/principal findings: In this study, we found that the expression of FXR was decreased in the liver of infected mice as shown by western blot and RT-qPCR assays. Furthermore, hepatocyte-specific FXR-deficient mice (FXRflox/floxAlbCre, FXR-HKO) were generated and infected with ~16 cercariae of S. japonicum for five weeks. We found that FXR deficiency in hepatocytes promoted the progression of liver injury, aggravated weight loss and death caused by infection, and promoted inflammatory cytokines production, such as IL-6, IL-1β, TNF-α, IL-4, IL-10, and IL-13. Surprisingly, hepatic granulomas and fibrosis were not affected. In addition, using UPLC-MS/MS spectrometry, it was found that S. japonicum infection resulted in elevated bile acids in the liver of mice, which was more obvious in FXR-deficient mice. Meanwhile, autophagy was induced in littermate control mice due to the infection, but it was significantly decreased in FXR-HKO mice.
Conclusions/significance: All these findings suggest that FXR deficiency in hepatocytes disrupts bile acid homeostasis and inhibits autophagy, which may aggravate the damages of hepatocytes caused by S. japonicum infection. It highlights that FXR in hepatocytes plays a regulatory role in the progression of schistosomiasis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

