Bedaquiline-based treatment for extensively drug-resistant tuberculosis in South Africa: A cost-effectiveness analysis
- PMID: 35930574
- PMCID: PMC9355220
- DOI: 10.1371/journal.pone.0272770
Bedaquiline-based treatment for extensively drug-resistant tuberculosis in South Africa: A cost-effectiveness analysis
Abstract
Background: The treatment success rate of conventional anti-tuberculosis (TB) regimens for extensively drug-resistant TB (XDR-TB) is low, resulting in high morbidity and healthcare cost especially in the high TB burden countries. Recent clinical findings reported improved treatment outcomes of XDR-TB with the bedaquiline (BDQ)-based regimens. We aimed to evaluate the cost-effectiveness of BDQ-based treatment for XDR-TB from the perspective of the South Africa national healthcare provider.
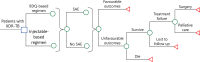
Methods: A 2-year decision-analytic model was designed to evaluate the clinical and economic outcomes of a hypothetical cohort of adult XDR-TB patients with (1) BDQ-based regimen and (2) injectable-based conventional regimen. The model inputs were retrieved from literature and public data. Base-case analysis and sensitivity analysis were performed. The primary model outputs included TB-related direct medical cost and disability-adjusted life years (DALYs).
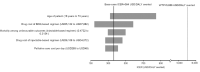
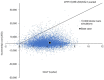
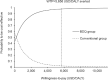
Results: In the base-case analysis, the BDQ group reduced 4.4152 DALYs with an incremental cost of USD1,606 when compared to the conventional group. The incremental cost per DALY averted (ICER) by the BDQ group was 364 USD/DALY averted. No influential factor was identified in the sensitivity analysis. In probabilistic sensitivity analysis, the BDQ group was accepted as cost-effective in 97.82% of the 10,000 simulations at a willingness-to-pay threshold of 5,656 USD/DALY averted (1× gross domestic product per capita in South Africa).
Conclusion: The BDQ-based therapy appeared to be cost-effective and showed a high probability to be accepted as the preferred cost-effective option for active XDR-TB treatment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organization. Global Tuberculosis Report 2021. Geneva: WHO; 2021. [cited 2022 March 15]. Available from: https://www.who.int/publications/i/item/9789240037021.
-
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis, Module 4: Treatment—Drug-Resistant Tuberculosis Treatment. Geneva: WHO; 2020. [cited 2022 January 16]. Available from: https://www.who.int/publications/i/item/9789240007048. - PubMed
-
- Ndjeka N, Conradie F, Schnippel K, Hughes J, Bantubani N, Ferreira H, et al. Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. The International Journal of Tuberculosis and Lung Disease. 2015;19(8):979–85. doi: 10.5588/ijtld.14.0944 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

