Trace metal stoichiometry of dissolved organic matter in the Amazon plume
- PMID: 35930637
- PMCID: PMC9355362
- DOI: 10.1126/sciadv.abm2249
Trace metal stoichiometry of dissolved organic matter in the Amazon plume
Abstract
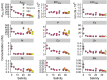
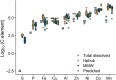
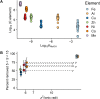
Dissolved organic matter (DOM) is a distinct component of Earth's hydrosphere and provides a link between the biogeochemical cycles of carbon, nutrients, and trace metals (TMs). Binding of TMs to DOM is thought to result in a TM pool with DOM-like biogeochemistry. Here, we determined elemental stoichiometries of aluminum, iron, copper, nickel, zinc, cobalt, and manganese associated with a fraction of the DOM pool isolated by solid-phase extraction at ambient pH (DOMSPE-amb) from the Amazon plume. We found that the rank order of TM stoichiometry within the DOMSPE-amb fraction was underpinned by the chemical periodicity of the TM. Furthermore, the removal of the TMSPE-amb pool at low salinity was related to the chemical hardness of the TM ion. Thus, the biogeochemistry of TMs bound to the DOMSPE-amb component in the Amazon plume was determined by the chemical nature of the TM and not by that of the DOMSPE-amb.
Figures



References
-
- Friedlingstein P., O’Sullivan M., Jones M. W., Andrew R. M., Hauck J., Olsen A., Peters G. P., Peters W., Pongratz J., Sitch S., le Quéré C., Canadell J. G., Ciais P., Jackson R. B., Alin S., Aragão L. E. O. C., Arneth A., Arora V., Bates N. R., Becker M., Benoit-Cattin A., Bittig H. C., Bopp L., Bultan S., Chandra N., Chevallier F., Chini L. P., Evans W., Florentie L., Forster P. M., Gasser T., Gehlen M., Gilfillan D., Gkritzalis T., Gregor L., Gruber N., Harris I., Hartung K., Haverd V., Houghton R. A., Ilyina T., Jain A. K., Joetzjer E., Kadono K., Kato E., Kitidis V., Korsbakken J. I., Landschützer P., Lefèvre N., Lenton A., Lienert S., Liu Z., Lombardozzi D., Marland G., Metzl N., Munro D. R., Nabel J. E. M. S., Nakaoka S. I., Niwa Y., O’Brien K., Ono T., Palmer P. I., Pierrot D., Poulter B., Resplandy L., Robertson E., Rödenbeck C., Schwinger J., Séférian R., Skjelvan I., Smith A. J. P., Sutton A. J., Tanhua T., Tans P. P., Tian H., Tilbrook B., van der Werf G., Vuichard N., Walker A. P., Wanninkhof R., Watson A. J., Willis D., Wiltshire A. J., Yuan W., Yue X., Zaehle S., Global carbon budget 2020. Earth Syst. Sci. Data 12, 3269–3340 (2020).
-
- D. A. Hansell, C. A. Carlson, Biogeochemistry of Marine Dissolved Organic Matter (Elsevier, ed. 2, 2015).
-
- Zakem E. J., Levine N. M., Systematic variation in marine dissolved organic matter stoichiometry and remineralization ratios as a function of lability. Global Biogeochem. Cycles 33, 1389–1407 (2019).
-
- Ksionzek K. B., Lechtenfeld O. J., McCallister S. L., Schmitt-Kopplin P., Geuer J. K., Geibert W., Koch B. P., Dissolved organic sulfur in the ocean: Biogeochemistry of a petagram inventory. Science 354, 456–459 (2016). - PubMed
LinkOut - more resources
Full Text Sources

