Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes
- PMID: 35930640
- PMCID: PMC9355358
- DOI: 10.1126/sciadv.abn4002
Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes
Abstract
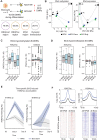
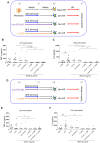
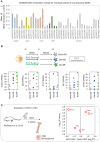
Trained immunity describes the capacity of innate immune cells to develop heterologous memory in response to certain exogenous exposures. This phenomenon mediates, at least in part, the beneficial off-target effects of the BCG vaccine. Using an in vitro model of trained immunity, we show that BCG exposure induces a persistent change in active histone modifications, DNA methylation, transcription, and adenosine-to-inosine RNA modification in human monocytes. By profiling DNA methylation of circulating monocytes from infants in the MIS BAIR clinical trial, we identify a BCG-associated DNA methylation signature that persisted more than 12 months after neonatal BCG vaccination. Genes associated with this epigenetic signature are involved in viral response pathways, consistent with the reported off-target protection against viral infections in neonates, adults, and the elderly. Our findings indicate that the off-target effects of BCG in infants are accompanied by epigenetic remodeling of circulating monocytes that lasts more than 1 year.
Figures






References
-
- Kleinnijenhuis J., Quintin J., Preijers F., Joosten L. A. B., Ifrim D. C., Saeed S., Jacobs C., van Loenhout J., de Jong D., Stunnenberg H. G., Xavier R. J., van der Meer J. W. M., van Crevel R., Netea M. G., Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. U.S.A. 109, 17537–17542 (2012). - PMC - PubMed
-
- Quintin J., Saeed S., Martens J. H. A., Giamarellos-Bourboulis E. J., Ifrim D. C., Logie C., Jacobs L., Jansen T., Kullberg B. J., Wijmenga C., Joosten L. A. B., Xavier R. J., van der Meer J. W. M., Stunnenberg H. G., Netea M. G., Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 (2012). - PMC - PubMed
-
- Arts R. J., Novakovic B., Horst R. T., Carvalho A., Bekkering S., Lachmandas E., Rodrigues F., Silvestre R., Cheng S.-C., Wang S.-Y., Habibi E., Gonçalves L. G., Mesquita I., Cunha C., van Laarhoven A., van de Veerdonk F. L., Williams D. L., van der Meer J. W. M., Logie C., O’Neill L. A., Dinarello C. A., Riksen N. P., van Crevel R., Clish C., Notebaart R. A., Joosten L. A. B., Stunnenberg H. G., Xavier R. J., Netea M. G., Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24, 807–819 (2016). - PMC - PubMed
-
- Bekkering S., Arts R. J. W., Novakovic B., Kourtzelis I., van der Heijden C. D. C. C., Li Y., Popa C. D., Horst R. T., van Tuijl J., Netea-Maier R. T., van de Veerdonk F. L., Chavakis T., Joosten L. A. B., van der Meer J. W. M., Stunnenberg H., Riksen N. P., Netea M. G., Metabolic induction of trained immunity through the mevalonate pathway. Cell 172, 135–146.e9 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

