Size-dependent activation of CAR-T cells
- PMID: 35930653
- PMCID: PMC9678385
- DOI: 10.1126/sciimmunol.abl3995
Size-dependent activation of CAR-T cells
Abstract
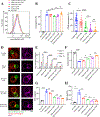
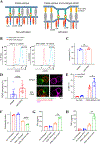
As the targets of chimeric antigen receptor (CAR)-T cells expand to a variety of cancers, autoimmune diseases, viral infections, and fibrosis, there is an increasing demand for identifying new antigens and designing new CARs that can be effectively activated. However, the rational selection of antigens and the design of CARs are limited by a lack of knowledge regarding the molecular mechanism by which CARs are activated by antigens. Here, we present data supporting a "size exclusion" model explaining how antigen signals are transmitted across the plasma membrane to activate the intracellular domains of CARs. In this model, antigen engagement with CAR results in a narrow intermembrane space that physically excludes CD45, a bulky phosphatase, out of the CAR zone, thus favoring CAR phosphorylation by kinases, which further triggers downstream pathways leading to T cell activation. Aligned with this model, increasing the size of CAR extracellular domains diminished CAR-T activation both in vitro and in a mouse lymphoma model; membrane-proximal epitopes activated CAR-Ts better than membrane-distal epitopes. Moreover, increasing the size of CD45 by antibody conjugation enhanced the activation of CARs that recognize membrane-distal epitopes. Consistently, CAR-Ts expressing CD45RABC, the larger isoform, were activated to a higher level than those expressing a smaller isoform CD45RO. Together, our work revealed that CAR-T activation depends on the size difference between the CAR-antigen pair and CD45; the size of CAR, antigen, and CD45 can thus be targets for tuning CAR-T activation.
Conflict of interest statement
Competing interests:
CH provides consulting for GlaxoSmithKine and PACT Pharma, holds NIH patents and royalties in the field of immunotherapy and cell therapy, and receives research funding from Neogene Therapeutics and T-Cure Bioscience. XS is a co-applicant for a provisional patent on CAR, which is not based on the specific results in this manuscript.
Figures





References
-
- Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, McSweeney PA, Miklos DB, Pagel JM, Kersten MJ, Milpied N, Fung H, Topp MS, Houot R, Beitinjaneh A, Peng W, Zheng L, Rossi JM, Jain RK, Rao AV, Reagan PM, KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med 382, 1331–1342 (2020). - PMC - PubMed
-
- Leibman RS, Richardson MW, Ellebrecht CT, Maldini CR, Glover JA, Secreto AJ, Kulikovskaya I, Lacey SF, Akkina SR, Yi Y, Shaheen F, Wang J, Dufendach KA, Holmes MC, Collman RG, Payne AS, Riley JL, Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLoS Pathog 13, e1006613 (2017). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous